Peroxisome Proliferator-Activated Receptor γ, but Not α or G-Protein Coupled Estrogen Receptor Drives Functioning of Postnatal Boar Testis-Next Generation Sequencing Analysis
- PMID: 34679887
- PMCID: PMC8532933
- DOI: 10.3390/ani11102868
Peroxisome Proliferator-Activated Receptor γ, but Not α or G-Protein Coupled Estrogen Receptor Drives Functioning of Postnatal Boar Testis-Next Generation Sequencing Analysis
Abstract
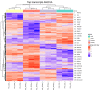
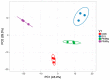
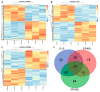
Porcine tissue gene expression is highly similar to the expression of homologous genes in humans. Based on this fact, the studies on porcine tissues can be employed to understand human physiology and to predict or treat diseases. Our prior studies clearly showed that there was a regulatory partnership of the peroxisome proliferator-activated receptor (PPAR) and the G-protein coupled membrane estrogen receptor (GPER) that relied upon the tumorigenesis of human and mouse testicular interstitial cells, as well as the PPAR-estrogen related receptor and GPER-xenoestrogen relationships which affected the functional status of immature boar testes. The main objective of this study was to identify the biological processes and signaling pathways governed by PPARα, PPARγ and GPER in the immature testes of seven-day-old boars after pharmacological receptor ligand treatment. Boar testicular tissues were cultured in an organotypic system with the respective PPARα, PPARγ or GPER antagonists. To evaluate the effect of the individual receptor deprivation in testicular tissue on global gene expression, Next Generation Sequencing was performed. Bioinformatic analysis revealed 382 transcripts with altered expression. While tissues treated with PPARα or GPER antagonists showed little significance in the enrichment analysis, the antagonists challenged with the PPARγ antagonist displayed significant alterations in biological processes such as: drug metabolism, adhesion and tubule development. Diverse disruption in the Notch signaling pathway was also observed. The findings of our study proposed that neither PPARα nor GPER, but PPARγ alone seemed to be the main player in the regulation of boar testes functioning during early the postnatal developmental window.
Keywords: G-protein coupled estrogen receptor; Next Generation Sequencing; boar; peroxisome proliferator-regulated receptor; testes.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
Similar articles
-
The meaning of non-classical estrogen receptors and peroxisome proliferator-activated receptor for boar Leydig cell of immature testis.Acta Histochem. 2020 Apr;122(3):151526. doi: 10.1016/j.acthis.2020.151526. Epub 2020 Feb 22. Acta Histochem. 2020. PMID: 32094002
-
Regulation of steroidogenic function of mouse Leydig cells: G-coupled membrane estrogen receptor and peroxisome proliferator-activated receptor partnership.J Physiol Pharmacol. 2018 Jun;69(3). doi: 10.26402/jpp.2018.3.04. Epub 2018 Aug 22. J Physiol Pharmacol. 2018. PMID: 30149370
-
Modulatory Effects of Estradiol and Its Mixtures with Ligands of GPER and PPAR on MAPK and PI3K/Akt Signaling Pathways and Tumorigenic Factors in Mouse Testis Explants and Mouse Tumor Leydig Cells.Biomedicines. 2022 Jun 12;10(6):1390. doi: 10.3390/biomedicines10061390. Biomedicines. 2022. PMID: 35740412 Free PMC article.
-
Role of GPER-Mediated Signaling in Testicular Functions and Tumorigenesis.Cells. 2020 Sep 17;9(9):2115. doi: 10.3390/cells9092115. Cells. 2020. PMID: 32957524 Free PMC article. Review.
-
GPER Signaling in Spermatogenesis and Testicular Tumors.Front Endocrinol (Lausanne). 2014 Mar 6;5:30. doi: 10.3389/fendo.2014.00030. eCollection 2014. Front Endocrinol (Lausanne). 2014. PMID: 24639669 Free PMC article. Review.
Cited by
-
Nuclear and Membrane Receptors for Sex Steroids Are Involved in the Regulation of Delta/Serrate/LAG-2 Proteins in Rodent Sertoli Cells.Int J Mol Sci. 2022 Feb 18;23(4):2284. doi: 10.3390/ijms23042284. Int J Mol Sci. 2022. PMID: 35216398 Free PMC article.
References
-
- Wernersson R., Schierup M.H., Jørgensen F.G., Gorodkin J., Panitz F., Staerfeldt H.H., Christensen O.F., Mailund T., Hornshøj H., Klein A., et al. Pigs in sequence space: A 0.66X coverage pig genome survey based on shotgun sequencing. BMC Genom. 2005;10:70. doi: 10.1186/1471-2164-6-70. - DOI - PMC - PubMed
-
- Pontelo T.P., Miranda J.R., Felix M.A.R., Pereira B.A., da Silva W.E., Avelar G.F., Mariano F.C.M.Q., Guimarães G.C., Zangeronimo M.G. Histological characteristics of the gonads of pig fetuses and their relationship with fetal anatomical measurements. Res. Vet. Sci. 2018;117:28–36. doi: 10.1016/j.rvsc.2017.11.005. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

