Interactions of HMGB Proteins with the Genome and the Impact on Disease
- PMID: 34680084
- PMCID: PMC8533419
- DOI: 10.3390/biom11101451
Interactions of HMGB Proteins with the Genome and the Impact on Disease
Abstract
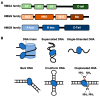
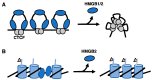
High Mobility Group Box (HMGB) proteins are small architectural DNA binding proteins that regulate multiple genomic processes such as DNA damage repair, nucleosome sliding, telomere homeostasis, and transcription. In doing so they control both normal cellular functions and impact a myriad of disease states, including cancers and autoimmune diseases. HMGB proteins bind to DNA and nucleosomes to modulate the local chromatin environment, which facilitates the binding of regulatory protein factors to the genome and modulates higher order chromosomal organization. Numerous studies over the years have characterized the structure and function of interactions between HMGB proteins and DNA, both biochemically and inside cells, providing valuable mechanistic insight as well as evidence these interactions influence pathological processes. This review highlights recent studies supporting the roles of HMGB1 and HMGB2 in global organization of the genome, as well as roles in transcriptional regulation and telomere maintenance via interactions with G-quadruplex structures. Moreover, emerging models for how HMGB proteins function as RNA binding proteins are presented. Nuclear HMGB proteins have broad regulatory potential to impact numerous aspects of cellular metabolism in normal and disease states.
Keywords: HMG protein; HMGB; architectural protein; chromatin; genome organization.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the writing of the manuscript.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

