Revisiting Jatropha curcas Monomeric Esterase: A Dienelactone Hydrolase Compatible with the Electrostatic Catapult Model
- PMID: 34680119
- PMCID: PMC8533429
- DOI: 10.3390/biom11101486
Revisiting Jatropha curcas Monomeric Esterase: A Dienelactone Hydrolase Compatible with the Electrostatic Catapult Model
Abstract
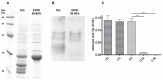
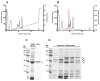
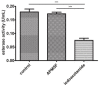
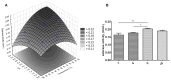
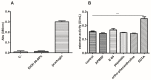
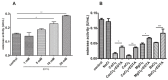
Jatropha curcas contains seeds with a high oil content, suitable for biodiesel production. After oil extraction, the remaining mass can be a rich source of enzymes. However, data from the literature describing physicochemical characteristics for a monomeric esterase from the J. curcas seed did not fit the electrostatic catapult model for esterases/lipases. We decided to reevaluate this J. curcas esterase and extend its characterization to check this apparent discrepancy and gain insights into the enzyme's potential as a biocatalyst. After anion exchange chromatography and two-dimensional gel electrophoresis, we identified the enzyme as belonging to the dienelactone hydrolase family, characterized by a cysteine as the nucleophile in the catalytic triad. The enzyme displayed a basic optimum hydrolysis pH of 9.0 and an acidic pI range, in contrast to literature data, making it well in line with the electrostatic catapult model. Furthermore, the enzyme showed low hydrolysis activity in an organic solvent-containing medium (isopropanol, acetonitrile, and ethanol), which reverted when recovering in an aqueous reaction mixture. This enzyme can be a valuable tool for hydrolysis reactions of short-chain esters, useful for pharmaceutical intermediates synthesis, due to both its high hydrolytic rate in basic pH and its stability in an organic solvent.
Keywords: Jatropha curcas L.; dienelactone hydrolase; esterase; seed.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Ewunie G.A., Morken J., Lekang O.I., Yigezu Z.D. Factors Affecting the Potential of Jatropha Curcas for Sustainable Biodiesel Production: A Critical Review. Renew. Sustain. Energy Rev. 2021;137:110500. doi: 10.1016/j.rser.2020.110500. - DOI
-
- Abobatta W.F. Jatropha curcas, a Novel Crop for Developing the Marginal Lands. In: Basu C., editor. Biofuels and Biodiesel. 1st ed. Springer Nature; New York, NY, USA: Humana Press; New York, NY, USA: 2021. pp. 79–100. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

