Mitochondrial Dysfunction, Protein Misfolding and Neuroinflammation in Parkinson's Disease: Roads to Biomarker Discovery
- PMID: 34680141
- PMCID: PMC8534011
- DOI: 10.3390/biom11101508
Mitochondrial Dysfunction, Protein Misfolding and Neuroinflammation in Parkinson's Disease: Roads to Biomarker Discovery
Abstract
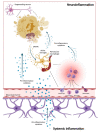
Parkinson's Disease (PD) is a highly prevalent neurodegenerative disease among older adults. PD neuropathology is marked by the progressive loss of the dopaminergic neurons of the substantia nigra pars compacta and the widespread accumulation of misfolded intracellular α-synuclein (α-syn). Genetic mutations and post-translational modifications, such as α-syn phosphorylation, have been identified among the multiple factors supporting α-syn accrual during PD. A decline in the clearance capacity of the ubiquitin-proteasome and the autophagy-lysosomal systems, together with mitochondrial dysfunction, have been indicated as major pathophysiological mechanisms of PD neurodegeneration. The accrual of misfolded α-syn aggregates into soluble oligomers, and the generation of insoluble fibrils composing the core of intraneuronal Lewy bodies and Lewy neurites observed during PD neurodegeneration, are ignited by the overproduction of reactive oxygen species (ROS). The ROS activate the α-syn aggregation cascade and, together with the Lewy bodies, promote neurodegeneration. However, the molecular pathways underlying the dynamic evolution of PD remain undeciphered. These gaps in knowledge, together with the clinical heterogeneity of PD, have hampered the identification of the biomarkers that may be used to assist in diagnosis, treatment monitoring, and prognostication. Herein, we illustrate the main pathways involved in PD pathogenesis and discuss their possible exploitation for biomarker discovery.
Keywords: DAMPs; alpha-synuclein; beta-amyloid (Aβ); cytokine; extracellular vesicles; inflammation; mitochondrial-derived vesicles; mitophagy; neurofilaments light chain (NfL); p-tau pathology.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the study design, data collection and analysis, preparation of the manuscript, or decision to publish.
Figures

References
-
- Ray Dorsey E., Elbaz A., Nichols E., Abd-Allah F., Abdelalim A., Adsuar J.C., Ansha M.G., Brayne C., Choi J.Y.J., Collado-Mateo D., et al. Global, regional, and national burden of Parkinson’s disease, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018;17:939–953. doi: 10.1016/S1474-4422(18)30295-3. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

