Progress on Ras/MAPK Signaling Research and Targeting in Blood and Solid Cancers
- PMID: 34680208
- PMCID: PMC8534156
- DOI: 10.3390/cancers13205059
Progress on Ras/MAPK Signaling Research and Targeting in Blood and Solid Cancers
Abstract
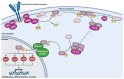
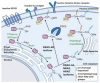
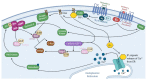
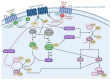
The mitogen-activated protein kinase (MAPK) pathway, consisting of the Ras-Raf-MEK-ERK signaling cascade, regulates genes that control cellular development, differentiation, proliferation, and apoptosis. Within the cascade, multiple isoforms of Ras and Raf each display differences in functionality, efficiency, and, critically, oncogenic potential. According to the NCI, over 30% of all human cancers are driven by Ras genes. This dysfunctional signaling is implicated in a wide variety of leukemias and solid tumors, both with and without viral etiology. Due to the strong evidence of Ras-Raf involvement in tumorigenesis, many have attempted to target the cascade to treat these malignancies. Decades of unsuccessful experimentation had deemed Ras undruggable, but recently, the approval of Sotorasib as the first ever KRas inhibitor represents a monumental breakthrough. This advancement is not without novel challenges. As a G12C mutant-specific drug, it also represents the issue of drug target specificity within Ras pathway; not only do many drugs only affect single mutational profiles, with few pan-inhibitor exceptions, tumor genetic heterogeneity may give rise to drug-resistant profiles. Furthermore, significant challenges in targeting downstream Raf, especially the BRaf isoform, lie in the paradoxical activation of wild-type BRaf by BRaf mutant inhibitors. This literature review will delineate the mechanisms of Ras signaling in the MAPK pathway and its possible oncogenic mutations, illustrate how specific mutations affect the pathogenesis of specific cancers, and compare available and in-development treatments targeting the Ras pathway.
Keywords: ATLL; ERK; MEK; Ras signaling; leukemia; solid tumors; viral oncogenesis.
Conflict of interest statement
All authors declare that they have no conflict of interest.
Figures

References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

