Treatment of Patients with Monoclonal Gammopathy of Clinical Significance
- PMID: 34680279
- PMCID: PMC8533809
- DOI: 10.3390/cancers13205131
Treatment of Patients with Monoclonal Gammopathy of Clinical Significance
Abstract
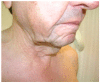
Monoclonal gammopathy of undetermined significance (MGUS) is defined as the presence of a monoclonal protein (M-protein) produced by a small amount of plasma cells. The majority of patients remain asymptomatic; however, a fraction of them develop clinical manifestations related to the monoclonal gammopathy despite not fulfilling criteria of multiple myeloma or other lymphoproliferative disorder. These patients constitute an emerging clinical issue coined as monoclonal gammopathy of clinical significance (MGCS). The mechanisms involved are poorly understood, and literature is scarce regarding management. The clinical spectrum involves symptoms related to renal, neurologic, skin, ocular, or bleeding manifestations, requiring a multidisciplinary approach. Treatment strategies rely on the basis of symptomatic disease and the M-protein isotype. In this review, we focus on MGCS other than renal, as the latter was earliest recognized and better known. We review the literature and discuss management from diagnosis to treatment based on illustrative cases from daily practice.
Keywords: MGCS; MGUS; bleeding; ocular; skin.
Conflict of interest statement
J.B.: Honoraria for lectures from Janssen, Celgene, Amgen, Takeda, and Oncopeptides. L.R.: Consulting fees from Amgen, Celgene, Sanofi, Janssen, and Takeda. C.F.d.L.: Advisory boards from Amgen, Janssen, and BMS; research grants from Janssen, BMS, Takeda, and Amgen; honoraria for lectures: BMS, Takeda, Sanofi, Amgen, Janssen, GSK, and Beigene. M.T.C.: Honoraria from Amgen and Janssen. D.F.M. declares no conflict of interest. This review was presented by Joan Bladé in the 24th European Hematology Association Congress (Amsterdam, 14 June 2019).
Figures
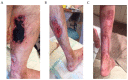
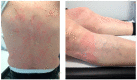
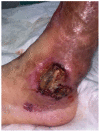
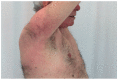
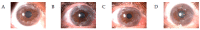
References
-
- Rajkumar S.V., Dimopoulos M.A., Palumbo A., Blade J., Merlini G., Mateos M.-V., Kumar S., Hillengass J., Kastritis E., Richardson P., et al. International Myeloma Working Group Updated Criteria for the Diagnosis of Multiple Myeloma. Lancet Oncol. 2014;15:e538–e548. doi: 10.1016/S1470-2045(14)70442-5. - DOI - PubMed
-
- Owen R.G., Treon S.P., Al-Katib A., Fonseca R., Greipp P.R., McMaster M.L., Morra E., Pangalis G.A., San Miguel J.F., Branagan A.R., et al. Clinicopathological Definition of Waldenstrom’s Macroglobulinemia: Consensus Panel Recommendations from the Second International Workshop on Waldenstrom’s Macroglobulinemia. Semin. Oncol. 2003;30:110–115. doi: 10.1053/sonc.2003.50082. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

