The Current Landscape of NKT Cell Immunotherapy and the Hills Ahead
- PMID: 34680322
- PMCID: PMC8533824
- DOI: 10.3390/cancers13205174
The Current Landscape of NKT Cell Immunotherapy and the Hills Ahead
Abstract
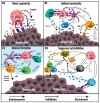
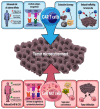
NKT cells are a specialized subset of lipid-reactive T lymphocytes that play direct and indirect roles in immunosurveillance and anti-tumor immunity. Preclinical studies have shown that NKT cell activation via delivery of exogenous glycolipids elicits a significant anti-tumor immune response. Furthermore, infiltration of NKT cells is associated with a good prognosis in several cancers. In this review, we aim to summarize the role of NKT cells in cancer as well as the current strategies and status of NKT cell immunotherapy. This review also examines challenges and future directions for improving the therapy.
Keywords: CAR-NKT cells; adoptive transfers; cancer; cancer vaccines; checkpoint inhibitors; iNKT cells; immunotherapy; natural killer T cells; type II NKT cells; α-galactosylceramide.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Mohan G., Hama A., Jijo A.J., Saradha Devi K.M., Narayanasamy A., Vellingiri B. Recent advances in radiotherapy and its associated side effects in cancer—A review. J. Basic Appl. Zool. 2019;80:14. doi: 10.1186/s41936-019-0083-5. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

