Comprehensive Analysis of VEGFR2 Expression in HPV-Positive and -Negative OPSCC Reveals Differing VEGFR2 Expression Patterns
- PMID: 34680369
- PMCID: PMC8533978
- DOI: 10.3390/cancers13205221
Comprehensive Analysis of VEGFR2 Expression in HPV-Positive and -Negative OPSCC Reveals Differing VEGFR2 Expression Patterns
Abstract
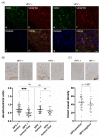
VEGF signaling regulated by the vascular endothelial growth factor receptor 2 (VEGFR2) plays a decisive role in tumor angiogenesis, initiation and progression in several tumors including HNSCC. However, the impact of HPV-status on the expression of VEGFR2 in OPSCC has not yet been investigated, although HPV oncoproteins E6 and E7 induce VEGF-expression. In a series of 56 OPSCC with known HPV-status, VEGFR2 expression patterns were analyzed both in blood vessels from tumor-free and tumor-containing regions and within tumor cells by immunohistochemistry using densitometry. Differences in subcellular colocalization of VEGFR2 with endothelial, tumor and stem cell markers were determined by double-immunofluorescence imaging. Immunohistochemical results were correlated with clinicopathological data. HPV-infection induces significant downregulation of VEGFR2 in cancer cells compared to HPV-negative tumor cells (p = 0.012). However, with respect to blood vessel supply, the intensity of VEGFR2 staining differed only in HPV-positive OPSCC and was upregulated in the blood vessels of tumor-containing regions (p < 0.0001). These results may suggest different routes of VEGFR2 signaling depending on the HPV-status of the OPSCC. While in HPV-positive OPSCC, VEGFR2 might be associated with increased angiogenesis, in HPV-negative tumors, an autocrine loop might regulate tumor cell survival and invasion.
Keywords: cancer stem cell; human papillomavirus; oropharyngeal squamous cell carcinoma; vascular endothelial growth factor receptor 2.
Conflict of interest statement
All other authors declare no conflict of interest.
Figures




References
-
- Chaturvedi A.K., Engels E.A., Pfeiffer R.M., Hernandez B.Y., Xiao W., Kim E., Jiang B., Goodman M.T., Sibug-Saber M., Cozen W., et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J. Clin. Oncol. 2011;29:4294–4301. doi: 10.1200/JCO.2011.36.4596. - DOI - PMC - PubMed
-
- CDC Cancers Associated with Human Papillomavirus, United States—2012–2016. USCS Data Brief, no 10. [(accessed on 8 February 2020)]; Available online: https://www.cdc.gov/cancer/uscs/about/data-briefs/no10-hpv-assoc-cancers....
Grants and funding
LinkOut - more resources
Full Text Sources

