Immune Responses against SARS-CoV-2-Questions and Experiences
- PMID: 34680460
- PMCID: PMC8533170
- DOI: 10.3390/biomedicines9101342
Immune Responses against SARS-CoV-2-Questions and Experiences
Abstract
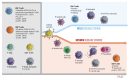
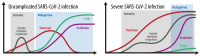
Understanding immune reactivity against SARS-CoV-2 is essential for coping with the COVID-19 pandemic. Herein, we discuss experiences and open questions about the complex immune responses to SARS-CoV-2. Some people react excellently without experiencing any clinical symptoms, they do not get sick, and they do not pass the virus on to anyone else ("sterilizing" immunity). Others produce antibodies and do not get COVID-19 but transmit the virus to others ("protective" immunity). Some people get sick but recover. A varying percentage develops respiratory failure, systemic symptoms, clotting disorders, cytokine storms, or multi-organ failure; they subsequently decease. Some develop long COVID, a new pathologic entity similar to fatigue syndrome or autoimmunity. In reality, COVID-19 is considered more of a systemic immune-vascular disease than a pulmonic disease, involving many tissues and the central nervous system. To fully comprehend the complex clinical manifestations, a profound understanding of the immune responses to SARS-CoV-2 is a good way to improve clinical management of COVID-19. Although neutralizing antibodies are an established approach to recognize an immune status, cellular immunity plays at least an equivalent or an even more important role. However, reliable methods to estimate the SARS-CoV-2-specific T cell capacity are not available for clinical routines. This deficit is important because an unknown percentage of people may exist with good memory T cell responsibility but a low number of or completely lacking peripheral antibodies against SARS-CoV-2. Apart from natural immune responses, vaccination against SARS-CoV-2 turned out to be very effective and much safer than naturally acquired immunity. Nevertheless, besides unwanted side effects of the currently available vector and mRNA preparations, concerns remain whether these vaccines will be strong enough to defeat the pandemic. Altogether, herein we discuss important questions, and try to give answers based on the current knowledge and preliminary data from our laboratories.
Keywords: SARS-CoV-2; T and B cell responses; development of the COVID-19 pandemic; immunity; inflammation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

