Inflammation in Duchenne Muscular Dystrophy-Exploring the Role of Neutrophils in Muscle Damage and Regeneration
- PMID: 34680483
- PMCID: PMC8533596
- DOI: 10.3390/biomedicines9101366
Inflammation in Duchenne Muscular Dystrophy-Exploring the Role of Neutrophils in Muscle Damage and Regeneration
Abstract
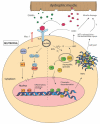
Duchenne muscular dystrophy (DMD) is a severe and progressive, X-linked, neuromuscular disorder caused by mutations in the dystrophin gene. In DMD, the lack of functional dystrophin protein makes the muscle membrane fragile, leaving the muscle fibers prone to damage during contraction. Muscle degeneration in DMD patients is closely associated with a prolonged inflammatory response, and while this is important to stimulate regeneration, inflammation is also thought to exacerbate muscle damage. Neutrophils are one of the first immune cells to be recruited to the damaged muscle and are the first line of defense during tissue injury or infection. Neutrophils can promote inflammation by releasing pro-inflammatory cytokines and compounds, including myeloperoxidase (MPO) and neutrophil elastase (NE), that lead to oxidative stress and are thought to have a role in prolonging inflammation in DMD. In this review, we provide an overview of the roles of the innate immune response, with particular focus on mechanisms used by neutrophils to exacerbate muscle damage and impair regeneration in DMD.
Keywords: DMD; Duchenne muscular dystrophy; inflammation; myeloperoxidase; neutrophil elastase; neutrophils.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Omairi S., Hau K.-L., Collins-Hooper H., Scott C., Vaiyapuri S., Torelli S., Montanaro F., Matsakas A., Patel K. Regulation of the dystrophin-associated glycoprotein complex composition by the metabolic properties of muscle fibres. Sci. Rep. 2019;9:2770. doi: 10.1038/s41598-019-39532-4. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

