Bilateral Adrenal Hyperplasia: Pathogenesis and Treatment
- PMID: 34680514
- PMCID: PMC8533142
- DOI: 10.3390/biomedicines9101397
Bilateral Adrenal Hyperplasia: Pathogenesis and Treatment
Abstract
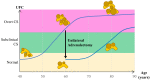
Bilateral adrenal hyperplasia is a rare cause of Cushing's syndrome. Micronodular adrenal hyperplasia, including the primary pigmented micronodular adrenal dysplasia (PPNAD) and the isolated micronodular adrenal hyperplasia (iMAD), can be distinguished from the primary bilateral macronodular adrenal hyperplasia (PBMAH) according to the size of the nodules. They both lead to overt or subclinical CS. In the latter case, PPNAD is usually diagnosed after a systematic screening in patients presenting with Carney complex, while for PBMAH, the diagnosis is often incidental on imaging. Identification of causal genes and genetic counseling also help in the diagnoses. This review discusses the last decades' findings on genetic and molecular causes of bilateral adrenal hyperplasia, including the several mechanisms altering the PKA pathway, the recent discovery of ARMC5, and the role of the adrenal paracrine regulation. Finally, the treatment of bilateral adrenal hyperplasia will be discussed, focusing on current data on unilateral adrenalectomy.
Keywords: ARMC5; Carney complex; Cushing’s syndrome; PKA pathway; PKRAR1A; bilateral adrenal hyperplasia; paracrine regulation; primary bilateral macronodular adrenal hyperplasia; primary pigmented micronodular adrenal; unilateral adrenalectomy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Stratakis C.A., Sarlis N., Kirschner L.S., Carney J.A., Doppman J.L., Nieman L.K., Chrousos G.P., Papanicolaou D.A. Paradoxical Response to Dexamethasone in the Diagnosis of Primary Pigmented Nodular Adrenocortical Disease. Ann. Intern. Med. 1999;131:585–591. doi: 10.7326/0003-4819-131-8-199910190-00006. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

