Adaptation by the Brown Planthopper to Resistant Rice: A Test of Female-Derived Virulence and the Role of Yeast-like Symbionts
- PMID: 34680677
- PMCID: PMC8539761
- DOI: 10.3390/insects12100908
Adaptation by the Brown Planthopper to Resistant Rice: A Test of Female-Derived Virulence and the Role of Yeast-like Symbionts
Abstract
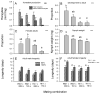
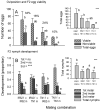
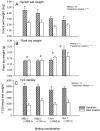
The adaptation by planthoppers to feed and develop on resistant rice is a challenge for pest management in Asia. We conducted a series of manipulative experiments with the brown planthopper (Nilaparvata lugens (Stål)) on the resistant rice variety IR62 (BPH3/BPH32 genes) to assess behavioral and bionomic changes in planthoppers exhibiting virulence adaptation. We also examined the potential role of yeast-like symbionts (YLS) in virulence adaptation by assessing progeny fitness (survival × reproduction) following controlled matings between virulent males or females and avirulent males or females, and by manipulating YLS densities in progeny through heat treatment. We found virulence-adapted planthoppers developed faster, grew larger, had adults that survived for longer, had female-biased progeny, and produced more eggs than non-selected planthoppers on the resistant variety. However, feeding capacity-as revealed through honeydew composition-remained inefficient on IR62, even after 20+ generations of exposure to the resistant host. Virulence was derived from both the male and female parents; however, females contributed more than males to progeny virulence. We found that YLS are essential for normal planthopper development and densities are highest in virulent nymphs feeding on the resistant host; however, we found only weak evidence that YLS densities contributed more to virulence. Virulence against IR62 in the brown planthopper, therefore, involves a complex of traits that encompass a series of behavioral, physiological, and genetic mechanisms, some of which are determined only by the female parent.
Keywords: BPH3; BPH32; Delphacidae; Heteroptera; Integrated Pest Management; endosymbionts; honeydew; host plant resistance; resistance management; rice breeding.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Bottrell D.G., Schoenly K.G. Resurrecting the ghost of green revolutions past: The brown planthopper as a recurring threat to high-yielding rice production in tropical Asia. J. Asia-Pac. Entomol. 2012;15:122–140. doi: 10.1016/j.aspen.2011.09.004. - DOI
-
- Horgan F.G., Crisol Martínez E., Stuart A.M., Bernal C.C., de Cima Martín E., Almazan M.L.P., Ramal A.F. Effects of vegetation strips, fertilizer levels and varietal resistance on the integrated management of arthropod biodiversity in a tropical rice ecosystem. Insects. 2019;10:328. doi: 10.3390/insects10100328. - DOI - PMC - PubMed
-
- Horgan F.G. Integrating gene deployment and crop management for improved rice resistance to Asian planthoppers. Crop. Prot. 2018;110:21–33. doi: 10.1016/j.cropro.2018.03.013. - DOI
-
- Kumar K., Kaur P., Kishore A., Vikal Y., Singh K., Neelam K. Recent advances in genomics-assisted breeding of brown planthopper (Nilaparvata lugens) resistance in rice (Oryza sativa) Plant. Breed. 2020;139:1052–1066. doi: 10.1111/pbr.12851. - DOI

