Drosophila Models for Charcot-Marie-Tooth Neuropathy Related to Aminoacyl-tRNA Synthetases
- PMID: 34680913
- PMCID: PMC8536177
- DOI: 10.3390/genes12101519
Drosophila Models for Charcot-Marie-Tooth Neuropathy Related to Aminoacyl-tRNA Synthetases
Abstract
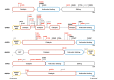
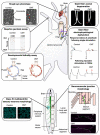
Aminoacyl-tRNA synthetases (aaRS) represent the largest cluster of proteins implicated in Charcot-Marie-Tooth neuropathy (CMT), the most common neuromuscular disorder. Dominant mutations in six aaRS cause different axonal CMT subtypes with common clinical characteristics, including progressive distal muscle weakness and wasting, impaired sensory modalities, gait problems and skeletal deformities. These clinical manifestations are caused by "dying back" axonal degeneration of the longest peripheral sensory and motor neurons. Surprisingly, loss of aminoacylation activity is not a prerequisite for CMT to occur, suggesting a gain-of-function disease mechanism. Here, we present the Drosophila melanogaster disease models that have been developed to understand the molecular pathway(s) underlying GARS1- and YARS1-associated CMT etiology. Expression of dominant CMT mutations in these aaRSs induced comparable neurodegenerative phenotypes, both in larvae and adult animals. Interestingly, recent data suggests that shared molecular pathways, such as dysregulation of global protein synthesis, might play a role in disease pathology. In addition, it has been demonstrated that the important function of nuclear YARS1 in transcriptional regulation and the binding properties of mutant GARS1 are also conserved and can be studied in D. melanogaster in the context of CMT. Taken together, the fly has emerged as a faithful companion model for cellular and molecular studies of aaRS-CMT that also enables in vivo investigation of candidate CMT drugs.
Keywords: Charcot–Marie–Tooth neuropathy; Drosophila melanogaster; aminoacyl-tRNA synthetases; disease-modeling.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Tooth H.H. The Peroneal Type of Progressive Muscular Atrophy. University of Cambridge; London, UK: 1886.
-
- Charcot J.M. Sur une forme paticuliere d’atrophie musuculaire progressive souvent familial, debutante par les pieds et les jambes et atteignant plus tard les mains. Rev. Med. Fr. 1886;6:97–138.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

