The Intrinsically Disordered Region in the Human STN1 OB-Fold Domain Is Important for Protecting Genome Stability
- PMID: 34681076
- PMCID: PMC8533325
- DOI: 10.3390/biology10100977
The Intrinsically Disordered Region in the Human STN1 OB-Fold Domain Is Important for Protecting Genome Stability
Abstract
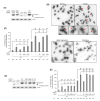
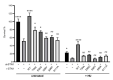
The mammalian CTC1-STN1-TEN1 (CST) complex is an ssDNA-binding protein complex that has emerged as an important player in protecting genome stability and preserving telomere integrity. Studies have shown that CST localizes at stalled replication forks and is critical for protecting the stability of nascent strand DNA. Recent cryo-EM analysis reveals that CST subunits possess multiple OB-fold domains that can form a decameric supercomplex. While considered to be RPA-like, CST acts distinctly from RPA to protect genome stability. Here, we report that while the OB domain of STN1 shares structural similarity with the OB domain of RPA32, the STN1-OB domain contains an intrinsically disordered region (IDR) that is important for maintaining genome stability under replication stress. Single mutations in multiple positions in this IDR, including cancer-associated mutations, cause genome instabilities that are elevated by replication stress and display reduced cellular viability and increased HU sensitivity. While IDR mutations do not impact CST complex formation or CST interaction with its binding partner RAD51, they diminish RAD51 foci formation when replication is perturbed. Interestingly, the IDR is critical for STN1-POLα interaction. Collectively, our results identify the STN1 IDR as an important element in regulating CST function in genome stability maintenance.
Keywords: CST; STN1; genome instability; replication stress.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Miyake Y., Nakamura M., Nabetani A., Shimamura S., Tamura M., Yonehara S., Saito M., Ishikawa F. RPA-like Mammalian Ctc1-Stn1-Ten1 Complex Binds to Single-Stranded DNA and Protects Telomeres Independently of the Pot1 Pathway. Mol. Cell. 2009;36:193–206. doi: 10.1016/j.molcel.2009.08.009. - DOI - PubMed
-
- Anderson B.H., Kasher P.R., Mayer J., Szynkiewicz M., Jenkinson E.M., Bhaskar S.S., Urquhart J.E., Daly S.B., Dickerson J.E., O’Sullivan J., et al. Mutations in CTC1, encoding conserved telomere maintenance component 1, cause Coats plus. Nat. Genet. 2012;44:338–342. doi: 10.1038/ng.1084. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

