Deciphering the Molecular Machinery-Influence of sE-Cadherin on Tumorigenic Traits of Prostate Cancer Cells
- PMID: 34681106
- PMCID: PMC8533516
- DOI: 10.3390/biology10101007
Deciphering the Molecular Machinery-Influence of sE-Cadherin on Tumorigenic Traits of Prostate Cancer Cells
Abstract
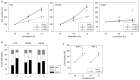
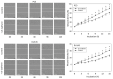
The serum level of soluble (s)E-cadherin is elevated in several malignancies, including prostate cancer (PCa). This study was designed to investigate the effects of sE-cadherin on the behavior of PCa cells in vitro, with the aim of identifying a potential therapeutic target. Growth as well as adhesive and motile behavior were evaluated in PC3, DU-145, and LNCaP cells. Flow cytometry was used to assess cell cycle phases and the surface expression of CD44 variants as well as α and β integrins. Confocal microscopy was utilized to visualize the distribution of CD44 variants within the cells. Western blot was applied to investigate expression of α3 and β1 integrins as well as cytoskeletal and adhesion proteins. Cell growth was significantly inhibited after exposure to 5 µg/mL sE-cadherin and was accompanied by a G0/G1-phase arrest. Adhesion of cells to collagen and fibronectin was mitigated, while motility was augmented. CD44v4, v5, and v7 expression was elevated while α3 and β1 integrins were attenuated. Blocking integrin α3 reduced cell growth and adhesion to collagen but increased motility. sE-cadherin therefore appears to foster invasive tumor cell behavior, and targeting it might serve as a novel and innovative concept to treat advanced PCa.
Keywords: cell growth; metastasis; migration; prostate cancer; sE-cadherin.
Conflict of interest statement
I.T.: advisory board: Sanofi, MSD, Pfizer; lecturer: Sanofi, Astellas, Janssen; congress travel support: Astellas, Janssen, Ipsen, Pfizer. A.T.: EUSP scholar.
Figures





References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

