Endothelial Thioredoxin-Interacting Protein Depletion Reduces Hemorrhagic Transformation in Hyperglycemic Mice after Embolic Stroke and Thrombolytic Therapy
- PMID: 34681207
- PMCID: PMC8537904
- DOI: 10.3390/ph14100983
Endothelial Thioredoxin-Interacting Protein Depletion Reduces Hemorrhagic Transformation in Hyperglycemic Mice after Embolic Stroke and Thrombolytic Therapy
Abstract
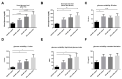
We hypothesize that endothelial-specific thioredoxin-interacting protein knock-out (EC-TXNIP KO) mice will be more resistant to the neurovascular damage (hemorrhagic-transformation-HT) associated with hyperglycemia (HG) in embolic stroke. Adult-male EC-TXNIP KO and wild-type (WT) littermate mice were injected with-streptozotocin (40 mg/kg, i.p.) for five consecutive days to induce diabetes. Four-weeks after confirming HG, mice were subjected to embolic middle cerebral artery occlusion (eMCAO) followed by tissue plasminogen activator (tPA)-reperfusion (10 mg/kg at 3 h post-eMCAO). After the neurological assessment, animals were sacrificed at 24 h for neurovascular stroke outcomes. There were no differences in cerebrovascular anatomy between the strains. Infarct size, edema, and HT as indicated by hemoglobin (Hb)-the content was significantly higher in HG-WT mice, with or without tPA-reperfusion, compared to normoglycemic WT mice. Hyperglycemic EC-TXNIP KO mice treated with tPA tended to show lower Hb-content, edema, infarct area, and less hemorrhagic score compared to WT hyperglycemic mice. EC-TXNIP KO mice showed decreased expression of inflammatory mediators, apoptosis-associated proteins, and nitrotyrosine levels. Further, vascular endothelial growth factor-A and matrix-metalloproteinases (MMP-9/MMP-3), which degrade junction proteins and increase blood-brain-barrier permeability, were decreased in EC-TXNIP KO mice. Together, these findings suggest that vascular-TXNIP could be a novel therapeutic target for neurovascular damage after stroke.
Keywords: embolic stroke; endothelial-specific TXNIP deletion; hemorrhagic transformation; hyperglycemia; tissue plasminogen activator.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures







References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

