Side Effects of mRNA-Based COVID-19 Vaccines among Young Adults (18-30 Years Old): An Independent Post-Marketing Study
- PMID: 34681273
- PMCID: PMC8696621
- DOI: 10.3390/ph14101049
Side Effects of mRNA-Based COVID-19 Vaccines among Young Adults (18-30 Years Old): An Independent Post-Marketing Study
Abstract
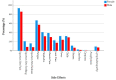
Young adults had been widely perceived as a low-risk group for COVID-19 severity; therefore, they were deprioritised within the mass vaccination strategies as their prognosis of COVID-19 infection is relatively more favourable than older age groups. On the other hand, vaccination of this demographic group is indispensable to achieve herd immunity. A cross-sectional survey-based study was used to evaluate the side effects of mRNA-based COVID-19 vaccines among university students in the Czech Republic. The validated questionnaire was delivered in a digital form, and it consisted of demographic data; COVID-19 vaccine-related anamnesis; and local, systemic, orofacial, and skin-related side effects' prevalence, onset, and duration. Out of the 539 included participants, 70.1% were females and 45.8% were <23 years old. The vast majority (95.2%) reported at least one side effect. The most common side effect was injection site pain (91.8%), followed by fatigue (62.5%), headache (36.4%), and muscle pain (34.9%). The majority of local side effects occurred after both doses (74.4%), while most systemic side effects occurred after the second dose only (56.2%). Most local (94.2%) and systemic (93.3%) side effects resolved within three days after vaccination. Females participants' adjusted odds ratio (AOR) showed they were 2.566 (CI 95%: 1.103-5.970) times more likely to experience post-vaccination side effects, and the participants who received two doses reported an increased AOR of 1.896 (0.708-5.077) for experiencing side effects. The results of this study imply that mRNA-based COVID-19 vaccines are highly probably safe for young adults, and further studies are required to investigate the role of medical anamnesis, prior COVID-19 infection, and gender in side effects incidence.
Keywords: BNT162 vaccine; COVID-19; Czech Republic; drug-related side effects and adverse reactions; mRNA-1273 vaccine; mass vaccination; phase IV; prevalence; young adult.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Di Gennaro F., Gualano G., Timelli L., Vittozzi P., Di Bari V., Libertone R., Cerva C., Pinnarelli L., Nisii C., Ianniello S., et al. Increase in Tuberculosis Diagnostic Delay during First Wave of the COVID-19 Pandemic: Data from an Italian Infectious Disease Referral Hospital. Antibiotics. 2021;10:272. doi: 10.3390/antibiotics10030272. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources