Sargahydroquinoic Acid Suppresses Hyperpigmentation by cAMP and ERK1/2-Mediated Downregulation of MITF in α-MSH-Stimulated B16F10 Cells
- PMID: 34681303
- PMCID: PMC8534327
- DOI: 10.3390/foods10102254
Sargahydroquinoic Acid Suppresses Hyperpigmentation by cAMP and ERK1/2-Mediated Downregulation of MITF in α-MSH-Stimulated B16F10 Cells
Abstract
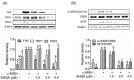
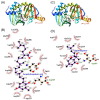
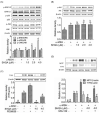
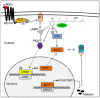
Hyperpigmentation diseases of the skin require topical treatment with depigmenting agents. We investigated the hypopigmented mechanisms of sargahydroquinoic acid (SHQA) in alpha-melanocyte-stimulating hormone (α-MSH)-stimulated B16F10 cells. SHQA reduced cellular tyrosinase (TYR) activity and melanin content in a concentration-dependent manner and attenuated the expression of TYR and tyrosinase-related protein 1 (TRP1), along with their transcriptional regulator, microphthalmia-associated transcription factor (MITF). SHQA also suppressed α-MSH-induced cellular production of cyclic adenosine monophosphate (cAMP), which inhibited protein kinase A (PKA)-dependent cAMP-responsive element-binding protein (CREB) activation. Docking simulation data showed a potential binding affinity of SHQA to the regulatory subunit RIIβ of PKA, which may also adversely affect PKA and CREB activation. Moreover, SHQA activated ERK1/2 signaling in B16F10 cells, stimulating the proteasomal degradation of MITF. These data suggest that SHQA ameliorated hyperpigmentation in α-MSH-stimulated B16F10 cells by downregulating MITF via PKA inactivation and ERK1/2 phosphorylation, indicating that SHQA is a potent therapeutic agent against skin hyperpigmentation disorders.
Keywords: ERK; MITF; cAMP; hyperpigmentation; melanin; sargahydroquinoic acid.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Sargaquinoic acid ameliorates hyperpigmentation through cAMP and ERK-mediated downregulation of MITF in α-MSH-stimulated B16F10 cells.Biomed Pharmacother. 2018 Aug;104:582-589. doi: 10.1016/j.biopha.2018.05.083. Epub 2018 May 25. Biomed Pharmacother. 2018. PMID: 29803170
-
Targeting phosphorylation circuits on CREB and CRTCs as the strategy to prevent acquired skin hyperpigmentation.Int J Biol Sci. 2024 Jan 1;20(1):312-330. doi: 10.7150/ijbs.86536. eCollection 2024. Int J Biol Sci. 2024. PMID: 38164184 Free PMC article.
-
Anti-Melanogenic Effects of Lilium lancifolium Root Extract via Downregulation of PKA/CREB and MAPK/CREB Signaling Pathways in B16F10 Cells.Plants (Basel). 2023 Oct 24;12(21):3666. doi: 10.3390/plants12213666. Plants (Basel). 2023. PMID: 37960022 Free PMC article.
-
Ssanghwa-tang, an oriental herbal cocktail, exerts anti-melanogenic activity by suppression of the p38 MAPK and PKA signaling pathways in B16F10 cells.BMC Complement Altern Med. 2013 Aug 28;13:214. doi: 10.1186/1472-6882-13-214. BMC Complement Altern Med. 2013. PMID: 23981281 Free PMC article.
-
[Regulation of melanogenesis: the role of cAMP and MITF].Postepy Hig Med Dosw (Online). 2012 Jan 30;66:33-40. Postepy Hig Med Dosw (Online). 2012. PMID: 22371403 Review. Polish.
Cited by
-
Potential Beneficial Effects of Sargassum spp. in Skin Aging.Mar Drugs. 2022 Aug 22;20(8):540. doi: 10.3390/md20080540. Mar Drugs. 2022. PMID: 36005543 Free PMC article. Review.
-
Syringetin Promotes Melanogenesis in B16F10 Cells.Int J Mol Sci. 2023 Jun 9;24(12):9960. doi: 10.3390/ijms24129960. Int J Mol Sci. 2023. PMID: 37373110 Free PMC article.
-
Anti-Melanogenesis Effect of Polysaccharide from Saussurea involucrata on Forskolin-Induced Melanogenesis in B16F10 Melanoma Cells.Nutrients. 2022 Nov 27;14(23):5044. doi: 10.3390/nu14235044. Nutrients. 2022. PMID: 36501075 Free PMC article.
-
Anti-tyrosinase and anti-melanogenic effects of piperine isolated from Piper nigrum on B16F10 mouse melanoma cells.Heliyon. 2024 Jun 22;10(12):e33423. doi: 10.1016/j.heliyon.2024.e33423. eCollection 2024 Jun 30. Heliyon. 2024. PMID: 39027450 Free PMC article.
-
Dietary Bioactive Compounds and Health.Foods. 2022 Aug 10;11(16):2395. doi: 10.3390/foods11162395. Foods. 2022. PMID: 36010394 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

