Biochemical and Immunological implications of Lutein and Zeaxanthin
- PMID: 34681572
- PMCID: PMC8535525
- DOI: 10.3390/ijms222010910
Biochemical and Immunological implications of Lutein and Zeaxanthin
Abstract
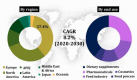
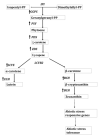
Throughout history, nature has been acknowledged for being a primordial source of various bioactive molecules in which human macular carotenoids are gaining significant attention. Among 750 natural carotenoids, lutein, zeaxanthin and their oxidative metabolites are selectively accumulated in the macular region of living beings. Due to their vast applications in food, feed, pharmaceutical and nutraceuticals industries, the global market of lutein and zeaxanthin is continuously expanding but chemical synthesis, extraction and purification of these compounds from their natural repertoire e.g., plants, is somewhat costly and technically challenging. In this regard microbial as well as microalgal carotenoids are considered as an attractive alternative to aforementioned challenges. Through the techniques of genetic engineering and gene-editing tools like CRISPR/Cas9, the overproduction of lutein and zeaxanthin in microorganisms can be achieved but the commercial scale applications of such procedures needs to be done. Moreover, these carotenoids are highly unstable and susceptible to thermal and oxidative degradation. Therefore, esterification of these xanthophylls and microencapsulation with appropriate wall materials can increase their shelf-life and enhance their application in food industry. With their potent antioxidant activities, these carotenoids are emerging as molecules of vital importance in chronic degenerative, malignancies and antiviral diseases. Therefore, more research needs to be done to further expand the applications of lutein and zeaxanthin.
Keywords: CRISPR/Cas9; antioxidants; bioavailability; genetic engineering; lutein binding protein; macular carotenoids.
Conflict of interest statement
The authors desire to endorse that there is no recognized conflict of interest allied with this publication and there has been no substantial economic funding for this work that could have predisposed its consequence.
Figures



References
-
- Bernstein P.S., Li B., Vachali P.P., Gorusupudi A., Shyam R., Henriksen B.S., Nolan J.M. Lutein, zeaxanthin, and meso-zeaxanthin: The basic and clinical science underlying carotenoid-based nutritional interventions against ocular disease. Prog. Retin. Eye Res. 2016;50:34–66. doi: 10.1016/j.preteyeres.2015.10.003. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

