Bisphenol a Induces Autophagy Defects and AIF-Dependent Apoptosis via HO-1 and AMPK to Degenerate N2a Neurons
- PMID: 34681608
- PMCID: PMC8535739
- DOI: 10.3390/ijms222010948
Bisphenol a Induces Autophagy Defects and AIF-Dependent Apoptosis via HO-1 and AMPK to Degenerate N2a Neurons
Abstract
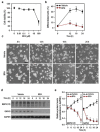
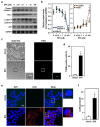
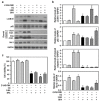
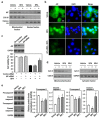
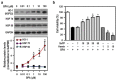
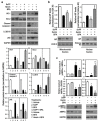
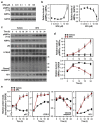
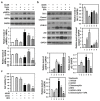
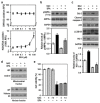
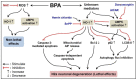
Bisphenol A (BPA) is an environmental contaminant widely suspected to be a neurological toxicant. Epidemiological studies have demonstrated close links between BPA exposure, pathogenetic brain degeneration, and altered neurobehaviors, considering BPA a risk factor for cognitive dysfunction. However, the mechanisms of BPA resulting in neurodegeneration remain unclear. Herein, cultured N2a neurons were subjected to BPA treatment, and neurotoxicity was assessed using neuronal viability and differentiation assays. Signaling cascades related to cellular self-degradation were also evaluated. BPA decreased cell viability and axon outgrowth (e.g., by down-regulating MAP2 and GAP43), thus confirming its role as a neurotoxicant. BPA induced neurotoxicity by down-regulating Bcl-2 and initiating apoptosis and autophagy flux inhibition (featured by nuclear translocation of apoptosis-inducing factor (AIF), light chain 3B (LC3B) aggregation, and p62 accumulation). Both heme oxygenase (HO)-1 and AMP-activated protein kinase (AMPK) up-regulated/activated by BPA mediated the molecular signalings involved in apoptosis and autophagy. HO-1 inhibition or AIF silencing effectively reduced BPA-induced neuronal death. Although BPA elicited intracellular oxygen free radical production, ROS scavenger NAC exerted no effect against BPA insults. These results suggest that BPA induces N2a neurotoxicity characterized by AIF-dependent apoptosis and p62-related autophagy defects via HO-1 up-regulation and AMPK activation, thereby resulting in neuronal degeneration.
Keywords: AMPK; Bisphenol A; apoptosis; autophagy; heme oxygenase-1; neurodegeneration.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
Similar articles
-
Activation of Autophagic Flux against Xenoestrogen Bisphenol-A-induced Hippocampal Neurodegeneration via AMP kinase (AMPK)/Mammalian Target of Rapamycin (mTOR) Pathways.J Biol Chem. 2015 Aug 21;290(34):21163-21184. doi: 10.1074/jbc.M115.648998. Epub 2015 Jul 2. J Biol Chem. 2015. Retraction in: J Biol Chem. 2020 Feb 28;295(9):2889. doi: 10.1074/jbc.W120.012895. PMID: 26139607 Free PMC article. Retracted.
-
Bisphenol A exhibits cytotoxic or genotoxic potential via oxidative stress-associated mitochondrial apoptotic pathway in murine macrophages.Food Chem Toxicol. 2018 Dec;122:215-224. doi: 10.1016/j.fct.2018.09.078. Epub 2018 Oct 9. Food Chem Toxicol. 2018. PMID: 30312649
-
Bisphenol A Modulates Autophagy and Exacerbates Chronic Kidney Damage in Mice.Int J Mol Sci. 2021 Jul 3;22(13):7189. doi: 10.3390/ijms22137189. Int J Mol Sci. 2021. PMID: 34281243 Free PMC article.
-
Heme oxygenase-1 enhances autophagy in podocytes as a protective mechanism against high glucose-induced apoptosis.Exp Cell Res. 2015 Oct 1;337(2):146-59. doi: 10.1016/j.yexcr.2015.04.005. Epub 2015 Apr 13. Exp Cell Res. 2015. PMID: 25882498 Review.
-
Role of Bisphenol A in Autophagy Modulation: Understanding the Molecular Concepts and Therapeutic Options.Mini Rev Med Chem. 2022;22(17):2213-2223. doi: 10.2174/1389557522666220214094055. Mini Rev Med Chem. 2022. PMID: 35156578 Review.
Cited by
-
Early-life bisphenol A exposure causes neuronal pyroptosis in juvenile and adult male rats through the NF-κB/IL-1β/NLRP3/caspase-1 signaling pathway: exploration of age and dose as effective covariates using an in vivo and in silico modeling approach.Mol Cell Biochem. 2025 Apr;480(4):2301-2330. doi: 10.1007/s11010-024-05039-4. Epub 2024 Jun 28. Mol Cell Biochem. 2025. PMID: 38941031 Free PMC article.
-
Effect of bisphenol A on the neurological system: a review update.Arch Toxicol. 2024 Jan;98(1):1-73. doi: 10.1007/s00204-023-03614-0. Epub 2023 Oct 19. Arch Toxicol. 2024. PMID: 37855918 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

