Expression Patterns of the Heat Shock Protein 90 (Hsp90) Gene Suggest Its Possible Involvement in Maintaining the Dormancy of Dinoflagellate Resting Cysts
- PMID: 34681714
- PMCID: PMC8538777
- DOI: 10.3390/ijms222011054
Expression Patterns of the Heat Shock Protein 90 (Hsp90) Gene Suggest Its Possible Involvement in Maintaining the Dormancy of Dinoflagellate Resting Cysts
Abstract
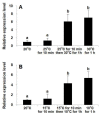
Heat shock protein 90 (Hsp90) is a highly conserved molecular chaperone functioning in cellular structural folding and conformational integrity maintenance and thus plays vital roles in a variety of biological processes. However, many aspects of these functions and processes remain to be fully elucidated, particularly for non-model organisms. Dinoflagellates are a group of eukaryotes that are exceedingly important in primary production and are responsible for the most harmful algal blooms (HABs) in aquatic ecosystems. The success of dinoflagellates in dominating the plankton community is undoubtedly pertinent to their remarkable adaptive strategies, characteristic of resting cyst production and broad tolerance to stresses of temperature and others. Therefore, this study was conducted to examine the putative roles of Hsp90 in the acclimation to temperature stress and life stage alterations of dinoflagellates. Firstly, we isolated the full-length cDNA of an Hsp90 gene (StHsp90) via RACE from the cosmopolitan HAB species Scrippsiella trochoidea and tracked its transcriptions in response to varied scenarios via real-time qPCR. The results indicated that StHsp90 displayed significant mRNA augment patterns, escalating during 180-min treatments, when the cells were exposed to elevated and lowered temperatures. Secondly, we observed prominently elevated StHsp90 transcriptions in the cysts that were stored at the cold and dark conditions compared to those in newly formed resting cysts and vegetative cells. Finally, and perhaps most importantly, we identified 29 entries of Hsp90-encoding genes with complete coding regions from a dinoflagellate-specific environmental cDNA library generated from marine sediment assemblages. The observed active transcription of these genes in sediment-buried resting cysts was fully supported by the qPCR results for the cold-stored resting cysts of S. trochoidea. Hsp90s expressions in both laboratory-raised and field-collected cysts collectively highlighted the possible involvement and engagement of Hsp90 chaperones in the resting stage persistence of dinoflagellates.
Keywords: Scrippsiella trochoidea; dinoflagellate; dormancy; environmental cDNA library; heat shock protein 90 (Hsp90); resting cysts; temperature stress.
Conflict of interest statement
All the authors declare that they have no conflicts of interest.
Figures



Similar articles
-
Probing the Energetic Metabolism of Resting Cysts under Different Conditions from Molecular and Physiological Perspectives in the Harmful Algal Blooms-Forming Dinoflagellate Scrippsiella trochoidea.Int J Mol Sci. 2021 Jul 7;22(14):7325. doi: 10.3390/ijms22147325. Int J Mol Sci. 2021. PMID: 34298944 Free PMC article.
-
Cloning and Partial Characterization of a Cold Shock Domain-Containing Protein Gene from the Dinoflagellate Scrippsiella trochoidea.J Eukaryot Microbiol. 2019 May;66(3):393-403. doi: 10.1111/jeu.12681. Epub 2018 Aug 29. J Eukaryot Microbiol. 2019. PMID: 30099808
-
Transcriptional Responses of the Heat Shock Protein 20 (Hsp20) and 40 (Hsp40) Genes to Temperature Stress and Alteration of Life Cycle Stages in the Harmful Alga Scrippsiella trochoidea (Dinophyceae).Biology (Basel). 2020 Nov 21;9(11):408. doi: 10.3390/biology9110408. Biology (Basel). 2020. PMID: 33233461 Free PMC article.
-
Cyst-forming dinoflagellates in a warming climate.Harmful Algae. 2020 Jan;91:101728. doi: 10.1016/j.hal.2019.101728. Epub 2019 Dec 20. Harmful Algae. 2020. PMID: 32057345 Free PMC article. Review.
-
[Heat shock proteins 90 kDa: diversity, structure, functions].Tsitologiia. 2010;52(11):893-910. Tsitologiia. 2010. PMID: 21268848 Review. Russian.
Cited by
-
Broad active metabolic pathways, autophagy, and antagonistic hormones regulate dinoflagellate cyst dormancy in marine sediments.Sci Adv. 2025 Feb 7;11(6):eads7789. doi: 10.1126/sciadv.ads7789. Epub 2025 Feb 7. Sci Adv. 2025. PMID: 39919173 Free PMC article.
References
-
- Efeoğlu B. Heat shock proteins and heat shock response in plants. GUJ Sci. 2009;22:67–75.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

