Photophysical Properties of BADAN Revealed in the Study of GGBP Structural Transitions
- PMID: 34681772
- PMCID: PMC8540541
- DOI: 10.3390/ijms222011113
Photophysical Properties of BADAN Revealed in the Study of GGBP Structural Transitions
Abstract
The fluorescent dye BADAN (6-bromoacetyl-2-dimetylaminonaphtalene) is widely used in various fields of life sciences, however, the photophysical properties of BADAN are not fully understood. The study of the spectral properties of BADAN attached to a number of mutant forms of GGBP, as well as changes in its spectral characteristics during structural changes in proteins, allowed to shed light on the photophysical properties of BADAN. It was shown that spectral properties of BADAN are determined by at least one non-fluorescent and two fluorescent isomers with overlapping absorbing bands. It was found that BADAN fluorescence is determined by the unsolvated "PICT" (planar intramolecular charge transfer state) and solvated "TICT" (twisted intramolecular charge transfer state) excited states. While "TICT" state can be formed both as a result of the "PICT" state solvation and as a result of light absorption by the solvated ground state of the dye. BADAN fluorescence linked to GGBP/H152C apoform is quenched by Trp 183, but this effect is inhibited by glucose intercalation. New details of the changes in the spectral characteristics of BADAN during the unfolding of the protein apo and holoforms have been obtained.
Keywords: BADAN (6-bromoacetyl-2-dimetylaminonaphtalene) spectroscopy; apo- and holo-forms of GGBP (D-glucose/D-galactose-binding protein); pathways of GGBP unfolding.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






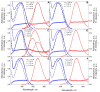
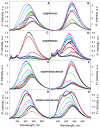

References
-
- Artyukhov V.Y., Zharkova O.M., Morozova Y.P. Complexing and photoprocesses in a PRODAN molecule. Russ. Phys. J. 2004;47:1172–1177. doi: 10.1007/s11182-005-0049-6. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

