Reversal Activity and Toxicity of Heparin-Binding Copolymer after Subcutaneous Administration of Enoxaparin in Mice
- PMID: 34681808
- PMCID: PMC8541278
- DOI: 10.3390/ijms222011149
Reversal Activity and Toxicity of Heparin-Binding Copolymer after Subcutaneous Administration of Enoxaparin in Mice
Abstract
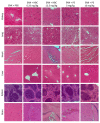
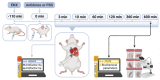
Uncontrolled bleeding after enoxaparin (ENX) is rare but may be life-threatening. The only registered antidote for ENX, protamine sulfate (PS), has 60% efficacy and can cause severe adverse side effects. We developed a diblock copolymer, heparin-binding copolymer (HBC), that reverses intravenously administered heparins. Here, we focused on the HBC inhibitory activity against subcutaneously administered ENX in healthy mice. BALB/c mice were subcutaneously injected with ENX at the dose of 5 mg/kg. After 110 min, vehicle, HBC (6.25 and 12.5 mg/kg), or PS (5 and 10 mg/kg) were administered into the tail vein. The blood was collected after 3, 10, 60, 120, 360, and 600 min after vehicle, HBC, or PS administration. The activities of antifactors Xa and IIa and biochemical parameters were measured. The main organs were collected for histological analysis. HBC at the lower dose reversed the effect of ENX on antifactor Xa activity for 10 min after antidote administration, whereas at the higher dose, HBC reversed the effect on antifactor Xa activity throughout the course of the experiment. Both doses of HBC completely reversed the effect of ENX on antifactor IIa activity. PS did not reverse antifactor Xa activity and partially reversed antifactor IIa activity. HBC modulated biochemical parameters. Histopathological analysis showed changes in the liver, lungs, and spleen of mice treated with HBC and in the lungs and heart of mice treated with PS. HBC administered in an appropriate dose might be an efficient substitute for PS to reverse significantly increased anticoagulant activity that may be connected with major bleeding in patients receiving ENX subcutaneously.
Keywords: anticoagulation; antidote; block copolymer; enoxaparin; heparins; macromolecules; mice; protamine; rodents; toxicity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Heparin-Binding Copolymer as a Complete Antidote for Low-Molecular-Weight Heparins in Rats.J Pharmacol Exp Ther. 2020 Apr;373(1):51-61. doi: 10.1124/jpet.119.262931. Epub 2020 Jan 14. J Pharmacol Exp Ther. 2020. PMID: 31937564
-
Heparin-binding copolymer reverses effects of unfractionated heparin, enoxaparin, and fondaparinux in rats and mice.Transl Res. 2016 Nov;177:98-112.e10. doi: 10.1016/j.trsl.2016.06.009. Epub 2016 Jul 5. Transl Res. 2016. PMID: 27456749
-
Thrombin generation assays are superior to traditional tests in assessing anticoagulation reversal in vitro.Thromb Haemost. 2008 Aug;100(2):350-5. Thromb Haemost. 2008. PMID: 18690358
-
Andexanet Alfa, the Possible Alternative to Protamine for Reversal of Unfractionated Heparin.Ann Pharmacother. 2021 Feb;55(2):261-264. doi: 10.1177/1060028020943160. Epub 2020 Jul 15. Ann Pharmacother. 2021. PMID: 32667214 Review.
-
[Reversal for heparins and new anticoagulant treatments].Ann Fr Anesth Reanim. 2013 Jan;32(1):37-49. doi: 10.1016/j.annfar.2012.10.034. Epub 2012 Dec 27. Ann Fr Anesth Reanim. 2013. PMID: 23273505 Review. French.
Cited by
-
Monitoring of Anticoagulant Activity of Dabigatran and Rivaroxaban in the Presence of Heparins.J Clin Med. 2022 Apr 16;11(8):2236. doi: 10.3390/jcm11082236. J Clin Med. 2022. PMID: 35456329 Free PMC article.
References
-
- Atiq F., van den Bemt P.M., Leebeek F.W., van Gelder T., Versmissen J. A systematic review on the accumulation of prophylactic dosages of low-molecular-weight heparins (LMWHs) in patients with renal insufficiency. Eur. J. Clin. Pharm. 2015;71:921–929. doi: 10.1007/s00228-015-1880-5. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

