Liquid Biopsies for Molecular Biology-Based Radiotherapy
- PMID: 34681925
- PMCID: PMC8538046
- DOI: 10.3390/ijms222011267
Liquid Biopsies for Molecular Biology-Based Radiotherapy
Abstract
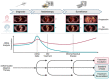
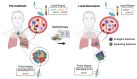
Molecular alterations drive cancer initiation and evolution during development and in response to therapy. Radiotherapy is one of the most commonly employed cancer treatment modalities, but radiobiologic approaches for personalizing therapy based on tumor biology and individual risks remain to be defined. In recent years, analysis of circulating nucleic acids has emerged as a non-invasive approach to leverage tumor molecular abnormalities as biomarkers of prognosis and treatment response. Here, we evaluate the roles of circulating tumor DNA and related analyses as powerful tools for precision radiotherapy. We highlight emerging work advancing liquid biopsies beyond biomarker studies into translational research investigating tumor clonal evolution and acquired resistance.
Keywords: biomarkers; circulating tumor DNA; liquid biopsies; precision oncology; radiation biology; treatment resistance; tumor evolution.
Conflict of interest statement
E.S.B. declares no competing interests. E.J.M. has served as a paid consultant for DeciBio.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

