RRM1 Expression as a Prognostic Biomarker for Unresectable or Recurrent Biliary Tract Cancer Treated with Gemcitabine plus Cisplatin
- PMID: 34682775
- PMCID: PMC8538709
- DOI: 10.3390/jcm10204652
RRM1 Expression as a Prognostic Biomarker for Unresectable or Recurrent Biliary Tract Cancer Treated with Gemcitabine plus Cisplatin
Abstract
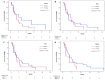
The combination of gemcitabine plus cisplatin (GP) is regarded as a first-line treatment for patients with unresectable or recurrent biliary tract cancer (BTC). Several proteins including human equilibrative nucleoside transporter-1 (hENT1), deoxycytidine kinase (DCK), cytidine deaminase (CDA), and ribonucleotide reductase subunit 1 (RRM1) are known to be involved in gemcitabine uptake and metabolism. This study was aimed to identify the predictive and prognostic values of these biomarkers in patients who treated with GP for advanced BTC. Tumor samples were obtained from 34 patients with unresectable or recurrent BTC who were treated with GP between August 2015 and February 2018. Intratumoral expression of hENT1, DCK, CDA and RRM1 was determined by immunohistochemistry and analyzed for association with chemotherapy response, progression-free survival (PFS) and overall survival (OS). Median OS was significantly longer in the RRM1-negative group than in the RRM1-positive (9.9 months vs. 5.9 months, p = 0.037). Multivariate adjustment analyses also demonstrated RRM1 expression as an independent prognostic factor for OS in patients treated with GP chemotherapy. Increased intratumoral expression of RRM1 on immunohistochemical staining may be a biomarker predicting poor survival in patients with GP chemotherapy for advanced BTC. Large-scale well-predefined prospective research is needed to validate the utility of biomarkers in clinical practice.
Keywords: biliary tract neoplasms; cisplatin; gemcitabine; ribonucleotide reductases.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Prognostic predictive values of gemcitabine sensitivity-related gene products for unresectable or recurrent biliary tract cancer treated with gemcitabine alone.World J Surg Oncol. 2013 May 27;11:117. doi: 10.1186/1477-7819-11-117. World J Surg Oncol. 2013. PMID: 23710668 Free PMC article.
-
Gemcitabine sensitivity factors, hENT1 and RRM1 as potential prognostic biomarker for advanced biliary tract cancer.Int J Clin Exp Med. 2014 Dec 15;7(12):5041-9. eCollection 2014. Int J Clin Exp Med. 2014. PMID: 25664003 Free PMC article.
-
Cytidine Deaminase as a Molecular Predictor of Gemcitabine Response in Patients with Biliary Tract Cancer.Oncology. 2015;89(6):345-50. doi: 10.1159/000439222. Epub 2015 Sep 30. Oncology. 2015. PMID: 26418006
-
Prognostic immunohistochemical biomarkers of chemotherapy efficacy in biliary tract cancer: A systematic review and meta-analysis.Crit Rev Oncol Hematol. 2019 Sep;141:82-94. doi: 10.1016/j.critrevonc.2019.06.001. Epub 2019 Jun 8. Crit Rev Oncol Hematol. 2019. PMID: 31255992
-
Human equilibrative nucleoside transporter 1 (hENT1) levels predict response to gemcitabine in patients with biliary tract cancer (BTC).Curr Cancer Drug Targets. 2011 Jan;11(1):123-9. doi: 10.2174/156800911793743600. Curr Cancer Drug Targets. 2011. PMID: 20578980 Review.
Cited by
-
The Roles of AGTRAP, ALKBH3, DIVERSIN, NEDD8 and RRM1 in Glioblastoma Pathophysiology and Prognosis.Biomedicines. 2024 Apr 22;12(4):926. doi: 10.3390/biomedicines12040926. Biomedicines. 2024. PMID: 38672281 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources

