Updates on the Current Treatments for Diabetic Retinopathy and Possibility of Future Oral Therapy
- PMID: 34682788
- PMCID: PMC8537579
- DOI: 10.3390/jcm10204666
Updates on the Current Treatments for Diabetic Retinopathy and Possibility of Future Oral Therapy
Abstract
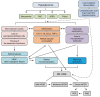
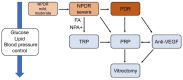
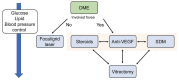
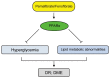
Diabetic retinopathy (DR) is a complication of diabetes and one of the leading causes of vision loss worldwide. Despite extensive efforts to reduce visual impairment, the prevalence of DR is still increasing. The initial pathophysiology of DR includes damage to vascular endothelial cells and loss of pericytes. Ensuing hypoxic responses trigger the expression of vascular endothelial growth factor (VEGF) and other pro-angiogenic factors. At present, the most effective treatment for DR and diabetic macular edema (DME) is the control of blood glucose levels. More advanced cases require laser, anti-VEGF therapy, steroid, and vitrectomy. Pan-retinal photocoagulation for non-proliferative diabetic retinopathy (NPDR) is well established and has demonstrated promising outcomes for preventing the progressive stage of DR. Furthermore, the efficacy of laser therapies such as grid and subthreshold diode laser micropulse photocoagulation (SDM) for DME has been reported. Vitrectomy has been performed for vitreous hemorrhage and tractional retinal detachment for patients with PDR. In addition, anti-VEGF treatment has been widely used for DME, and recently its potential to prevent the progression of PDR has been remarked. Even with these treatments, many patients with DR lose their vision and suffer from potential side effects. Thus, we need alternative treatments to address these limitations. In recent years, the relationship between DR, lipid metabolism, and inflammation has been featured. Research in diabetic animal models points to peroxisome proliferator-activated receptor alpha (PPARα) activation in cellular metabolism and inflammation by oral fenofibrate and/or pemafibrate as a promising target for DR. In this paper, we review the status of existing therapies, summarize PPARα activation therapies for DR, and discuss their potentials as promising DR treatments.
Keywords: anti-VEGF therapy; diabetic macula edema; diabetic retinopathy; fenofibrate; laser photocoagulation; pemafibrate; vitrectomy.
Conflict of interest statement
Kazuo Tsubota is CEO in Tsubota Laboratory, Inc. The remaining authors declare no conflict of interest.
Figures
Similar articles
-
The Evolving Treatment of Diabetic Retinopathy.Clin Ophthalmol. 2020 Mar 4;14:653-678. doi: 10.2147/OPTH.S236637. eCollection 2020. Clin Ophthalmol. 2020. PMID: 32184554 Free PMC article. Review.
-
Intravitreal Anti-Vascular Endothelial Growth Factor Agents for the Treatment of Diabetic Retinopathy: A Review of the Literature.Pharmaceutics. 2021 Jul 26;13(8):1137. doi: 10.3390/pharmaceutics13081137. Pharmaceutics. 2021. PMID: 34452097 Free PMC article. Review.
-
Diabetic Retinopathy: An Overview of Treatments.Indian J Endocrinol Metab. 2022 Mar-Apr;26(2):111-118. doi: 10.4103/ijem.ijem_480_21. Epub 2022 Jun 6. Indian J Endocrinol Metab. 2022. PMID: 35873941 Free PMC article. Review.
-
Gene Therapy in Diabetic Retinopathy and Diabetic Macular Edema: An Update.J Clin Med. 2025 May 6;14(9):3205. doi: 10.3390/jcm14093205. J Clin Med. 2025. PMID: 40364236 Free PMC article. Review.
-
Evolving strategies in the management of diabetic retinopathy.Middle East Afr J Ophthalmol. 2013 Oct-Dec;20(4):273-82. doi: 10.4103/0974-9233.119993. Middle East Afr J Ophthalmol. 2013. PMID: 24339676 Free PMC article. Review.
Cited by
-
Therapeutic roles of PPARα activation in ocular ischemic diseases.Histol Histopathol. 2023 Apr;38(4):391-401. doi: 10.14670/HH-18-542. Epub 2022 Oct 28. Histol Histopathol. 2023. PMID: 36305579 Review.
-
Current Treatments and Innovations in Diabetic Retinopathy and Diabetic Macular Edema.Pharmaceutics. 2022 Dec 29;15(1):122. doi: 10.3390/pharmaceutics15010122. Pharmaceutics. 2022. PMID: 36678750 Free PMC article. Review.
-
Association between Systemic Factors and Vitreous Fluid Cytokines in Proliferative Diabetic Retinopathy.J Clin Med. 2023 Mar 17;12(6):2354. doi: 10.3390/jcm12062354. J Clin Med. 2023. PMID: 36983353 Free PMC article.
-
The signaling pathways of traditional Chinese medicine in treating diabetic retinopathy.Front Pharmacol. 2023 Jun 19;14:1165649. doi: 10.3389/fphar.2023.1165649. eCollection 2023. Front Pharmacol. 2023. PMID: 37405050 Free PMC article. Review.
-
Diabetic retinopathy is a ceramidopathy reversible by anti-ceramide immunotherapy.Cell Metab. 2024 Jul 2;36(7):1521-1533.e5. doi: 10.1016/j.cmet.2024.04.013. Epub 2024 May 7. Cell Metab. 2024. PMID: 38718792 Free PMC article.
References
-
- Bourne R.R.A., Flaxman S.R., Braithwaite T., Cicinelli M.V., Das A., Jonas J.B., Keeffe J., Kempen J.H., Leasher J., Limburg H., et al. Magnitude, temporal trends, and projections of the global prevalence of blindness and distance and near vision impairment: A systematic review and meta-analysis. Lancet Glob. Health. 2017;5:e888–e897. doi: 10.1016/S2214-109X(17)30293-0. - DOI - PubMed
-
- Leasher J.L., Bourne R.R., Flaxman S.R., Jonas J.B., Keeffe J., Naidoo K., Pesudovs K., Price H., White R.A., Wong T.Y., et al. Global Estimates on the Number of People Blind or Visually Impaired by Diabetic Retinopathy: A Meta-analysis from 1990 to 2010. Diabetes Care. 2016;39:1643–1649. doi: 10.2337/dc15-2171. - DOI - PubMed
-
- Matuszewski W., Baranowska-Jurkun A., Stefanowicz-Rutkowska M.M., Modzelewski R., Pieczynski J., Bandurska-Stankiewicz E. Prevalence of Diabetic Retinopathy in Type 1 and Type 2 Diabetes Mellitus Patients in North-East Poland. Medicine. 2020;56:164. doi: 10.3390/medicina56040164. - DOI - PMC - PubMed
-
- Stewart M.W. Socioeconomic Cost of Diabetic Retinopathy and Therapy. In: Stewart M.W., editor. Diabetic Retinopathy: Current Pharmacologic Treatment and Emerging Strategies. Springer; Singapore: 2017. pp. 257–268. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

