Role of microRNAs in Disorders of Gut-Brain Interactions: Clinical Insights and Therapeutic Alternatives
- PMID: 34683162
- PMCID: PMC8541612
- DOI: 10.3390/jpm11101021
Role of microRNAs in Disorders of Gut-Brain Interactions: Clinical Insights and Therapeutic Alternatives
Abstract
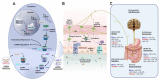
Disorders of gut-brain interactions (DGBIs) are heterogeneous in nature and intertwine with diverse pathophysiological mechanisms. Regular functioning of the gut requires complex coordinated interplay between a variety of gastrointestinal (GI) cell types and their functions are regulated by multiple mechanisms at the transcriptional, post-transcriptional, translational, and post-translational levels. MicroRNAs (miRNAs) are small non-coding RNA molecules that post-transcriptionally regulate gene expression by binding to specific mRNA targets to repress their translation and/or promote the target mRNA degradation. Dysregulation of miRNAs might impair gut physiological functions leading to DGBIs and gut motility disorders. Studies have shown miRNAs regulate gut functions such as visceral sensation, gut immune response, GI barrier function, enteric neuronal development, and GI motility. These biological processes are highly relevant to the gut where neuroimmune interactions are key contributors in controlling gut homeostasis and functional defects lead to DGBIs. Although extensive research has explored the pathophysiology of DGBIs, further research is warranted to bolster the molecular mechanisms behind these disorders. The therapeutic targeting of miRNAs represents an attractive approach for the treatment of DGBIs because they offer new insights into disease mechanisms and have great potential to be used in the clinic as diagnostic markers and therapeutic targets. Here, we review recent advances regarding the regulation of miRNAs in GI pacemaking cells, immune cells, and enteric neurons modulating pathophysiological mechanisms of DGBIs. This review aims to assess the impacts of miRNAs on the pathophysiological mechanisms of DGBIs, including GI dysmotility, impaired intestinal barrier function, gut immune dysfunction, and visceral hypersensitivity. We also summarize the therapeutic alternatives for gut microbial dysbiosis in DGBIs, highlighting the clinical insights and areas for further exploration. We further discuss the challenges in miRNA therapeutics and promising emerging approaches.
Keywords: functional dyspepsia; gastroparesis; gut barrier function; irritable bowel syndrome; miRNA therapeutics; neuroimmune interaction; serotonin; slow transit constipation; visceral hypersensitivity.
Conflict of interest statement
None of the authors has any conflict interest in relation to this paper.
Figures
Similar articles
-
Current Treatment Options and Therapeutic Insights for Gastrointestinal Dysmotility and Functional Gastrointestinal Disorders.Front Pharmacol. 2022 Jan 25;13:808195. doi: 10.3389/fphar.2022.808195. eCollection 2022. Front Pharmacol. 2022. PMID: 35145413 Free PMC article. Review.
-
Enterochromaffin Cells-Gut Microbiota Crosstalk: Underpinning the Symptoms, Pathogenesis, and Pharmacotherapy in Disorders of Gut-Brain Interaction.J Neurogastroenterol Motil. 2022 Jul 30;28(3):357-375. doi: 10.5056/jnm22008. Epub 2022 Jun 20. J Neurogastroenterol Motil. 2022. PMID: 35719046 Free PMC article. Review.
-
miR-10b-5p rescues leaky gut linked with gastrointestinal dysmotility and diabetes.United European Gastroenterol J. 2023 Oct;11(8):750-766. doi: 10.1002/ueg2.12463. Epub 2023 Sep 18. United European Gastroenterol J. 2023. PMID: 37723933 Free PMC article.
-
The gut microbiome in disorders of gut-brain interaction.Gut Microbes. 2024 Jan-Dec;16(1):2360233. doi: 10.1080/19490976.2024.2360233. Epub 2024 Jul 1. Gut Microbes. 2024. PMID: 38949979 Free PMC article. Review.
-
Uncovering the pathophysiology of irritable bowel syndrome by exploring the gut-brain axis: a narrative review.Ann Transl Med. 2021 Jul;9(14):1187. doi: 10.21037/atm-21-2779. Ann Transl Med. 2021. PMID: 34430628 Free PMC article. Review.
Cited by
-
Sustained Effectiveness and Safety of Therapeutic miR-10a/b in Alleviating Diabetes and Gastrointestinal Dysmotility without Inducing Cancer or Inflammation in Murine Liver and Colon.Int J Mol Sci. 2024 Feb 14;25(4):2266. doi: 10.3390/ijms25042266. Int J Mol Sci. 2024. PMID: 38396943 Free PMC article.
-
Functional Implications and Clinical Potential of MicroRNAs in Irritable Bowel Syndrome: A Concise Review.Dig Dis Sci. 2023 Jan;68(1):38-53. doi: 10.1007/s10620-022-07516-6. Epub 2022 May 4. Dig Dis Sci. 2023. PMID: 35507132 Free PMC article. Review.
-
Current Treatment Options and Therapeutic Insights for Gastrointestinal Dysmotility and Functional Gastrointestinal Disorders.Front Pharmacol. 2022 Jan 25;13:808195. doi: 10.3389/fphar.2022.808195. eCollection 2022. Front Pharmacol. 2022. PMID: 35145413 Free PMC article. Review.
-
Cross-Kingdom Interaction of miRNAs and Gut Microbiota with Non-Invasive Diagnostic and Therapeutic Implications in Colorectal Cancer.Int J Mol Sci. 2023 Jun 23;24(13):10520. doi: 10.3390/ijms241310520. Int J Mol Sci. 2023. PMID: 37445698 Free PMC article. Review.
-
Cytolethal distending toxin B inoculation leads to distinct gut microtypes and IBS-D-like microRNA-mediated gene expression changes in a rodent model.Gut Microbes. 2024 Jan-Dec;16(1):2293170. doi: 10.1080/19490976.2023.2293170. Epub 2023 Dec 18. Gut Microbes. 2024. PMID: 38108386 Free PMC article.
References
-
- Sperber A.D., Bangdiwala S.I., Drossman D.A., Ghoshal U.C., Simren M., Tack J., Whitehead W.E., Dumitrascu D.L., Fang X., Fukudo S., et al. Worldwide prevalence and burden of functional gastrointestinal disorders, results of rome foundation global study. Gastroenterology. 2021;160:99–114.e3. doi: 10.1053/j.gastro.2020.04.014. - DOI - PubMed
-
- Parkman H.P., Yates K., Hasler W.L., Nguyen L., Pasricha P.J., Snape W.J., Farrugia G., Koch K.L., Abell T.L., McCallum R.W., et al. Clinical features of idiopathic gastroparesis vary with sex, body mass, symptom onset, delay in gastric emptying, and gastroparesis severity. Gastroenterology. 2011;140:101–115. doi: 10.1053/j.gastro.2010.10.015. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

