The Nasopharyngeal, Ruminal, and Vaginal Microbiota and the Core Taxa Shared across These Microbiomes in Virgin Yearling Heifers Exposed to Divergent In Utero Nutrition during Their First Trimester of Gestation and in Pregnant Beef Heifers in Response to Mineral Supplementation
- PMID: 34683332
- PMCID: PMC8537542
- DOI: 10.3390/microorganisms9102011
The Nasopharyngeal, Ruminal, and Vaginal Microbiota and the Core Taxa Shared across These Microbiomes in Virgin Yearling Heifers Exposed to Divergent In Utero Nutrition during Their First Trimester of Gestation and in Pregnant Beef Heifers in Response to Mineral Supplementation
Abstract
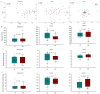
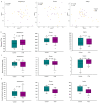
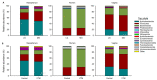
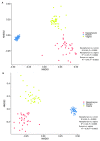
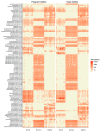
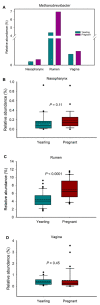
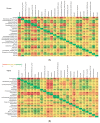
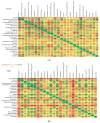
In the present study, we evaluated whether the nasopharyngeal, ruminal, and vaginal microbiota would diverge (1) in virgin yearling beef heifers (9 months old) due to the maternal restricted gain during the first trimester of gestation; and (2) in pregnant beef heifers in response to the vitamin and mineral (VTM) supplementation during the first 6 months of pregnancy. As a secondary objective, using the microbiota data obtained from these two cohorts of beef heifers managed at the same location and sampled at the same time, we performed a holistic assessment of the microbial ecology residing within the respiratory, gastrointestinal, and reproductive tract of cattle. Our 16S rRNA gene sequencing results revealed that both α and β-diversity of the nasopharyngeal, ruminal and vaginal microbiota did not differ between virgin heifers raised from dams exposed to either a low gain (targeted average daily gain of 0.28 kg/d, n = 22) or a moderate gain treatment (0.79 kg/d, n = 23) during the first 84 days of gestation. Only in the vaginal microbiota were there relatively abundant genera that were affected by maternal rate of gain during early gestation. Whilst there was no significant difference in community structure and diversity in any of the three microbiota between pregnant heifers received no VTM (n = 15) and VTM supplemented (n = 17) diets, the VTM supplementation resulted in subtle compositional alterations in the nasopharyngeal and ruminal microbiota. Although the nasopharyngeal, ruminal, and vaginal microbiota were clearly distinct, a total of 41 OTUs, including methanogenic archaea, were identified as core taxa shared across the respiratory, gastrointestinal, and reproductive tracts of both virgin and pregnant heifers.
Keywords: beef heifers; core taxa; maternal nutrition; nasopharyngeal microbiota; offspring; ruminal microbiota; vaginal microbiota.
Conflict of interest statement
The authors declare that they have no competing interest.
Figures
References
-
- Huws S.A., Creevey C.J., Oyama L.B., Mizrahi I., Denman S.E., Popova M., Muñoz-Tamayo R., Forano E., Waters S.M., Hess M., et al. Addressing Global Ruminant Agricultural Challenges Through Understanding the Rumen Microbiome: Past, Present, and Future. Front. Microbiol. 2018;9:2161. doi: 10.3389/fmicb.2018.02161. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

