Evidence of HIV-1 Genital Shedding after One Year of Antiretroviral Therapy in Females Recently Diagnosed in Bamako, Mali
- PMID: 34683485
- PMCID: PMC8538623
- DOI: 10.3390/microorganisms9102164
Evidence of HIV-1 Genital Shedding after One Year of Antiretroviral Therapy in Females Recently Diagnosed in Bamako, Mali
Abstract
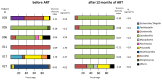
Little is known about the dynamic of HIV-1 shedding and resistance profiles in the female genital reservoir after antiretroviral therapy (ART) initiation in resource-limited countries (RLCs), which is critical for evaluating the residual sexual HIV-1 transmission risk. The present study aimed to evaluate the efficacy of 1 year duration ART at blood and genital levels in females newly diagnosed for HIV-1 from three centers in Bamako, Mali. Seventy-eight consenting females were enrolled at the time of their HIV-1 infection diagnosis. HIV-1 RNA loads (Abbott Real-Time HIV-1 assay) were tested in blood and cervicovaginal fluids (CVF) before and 12 months after ART initiation. Primary and acquired resistances to ART were evaluated by ViroseqTM HIV-1 genotyping assay. The vaginal microbiota was analyzed using IonTorrentTM NGS technology (Thermo Fisher Scientific). Proportions of primary drug resistance mutations in blood and CVF were 13.4% and 25%, respectively. Discrepant profiles were observed in 25% of paired blood/CVF samples. The acquired resistance rate was 3.1% in blood. At month 12, undetectable HIV-1 RNA load was reached in 84.6% and 75% of blood and CVF samples, respectively. A vaginal dysbiosis was associated with HIV RNA shedding. Our findings emphasize the need of reinforcing education to improve retention in care system, as well as the necessity of regular virological monitoring before and during ART and of implementing vaginal dysbiosis diagnosis and treatment in RLCs.
Keywords: HIV-1; Mali; antiretroviral therapy; genital reservoir; microbiota; resistance.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Impact of HIV-1 primary drug resistance on the efficacy of a first-line antiretroviral regimen in the blood of newly diagnosed individuals in Bamako, Mali.J Antimicrob Chemother. 2019 Jan 1;74(1):165-171. doi: 10.1093/jac/dky382. J Antimicrob Chemother. 2019. PMID: 30285106
-
Vaginal microbiome, antiretroviral concentrations, and HIV genital shedding in the setting of hormonal contraception initiation in Malawi.AIDS. 2023 Nov 15;37(14):2185-2190. doi: 10.1097/QAD.0000000000003686. Epub 2023 Aug 9. AIDS. 2023. PMID: 37877275 Free PMC article.
-
Twelve-Month Antiretroviral Therapy Suppresses Plasma and Genital Viral Loads but Fails to Alter Genital Levels of Cytokines, in a Cohort of HIV-Infected Rwandan Women.PLoS One. 2015 May 26;10(5):e0127201. doi: 10.1371/journal.pone.0127201. eCollection 2015. PLoS One. 2015. PMID: 26010956 Free PMC article.
-
Molecular biological assessment methods and understanding the course of the HIV infection.APMIS Suppl. 2003;(114):1-37. APMIS Suppl. 2003. PMID: 14626050 Review.
-
Study of the impact of HIV genotypic drug resistance testing on therapy efficacy.Verh K Acad Geneeskd Belg. 2001;63(5):447-73. Verh K Acad Geneeskd Belg. 2001. PMID: 11813503 Review.
Cited by
-
Longitudinal cervicovaginal microbiome and virome alterations during ART and discordant shedding in women living with HIV.Res Sq [Preprint]. 2024 Apr 15:rs.3.rs-4078561. doi: 10.21203/rs.3.rs-4078561/v1. Res Sq. 2024. PMID: 38699319 Free PMC article. Preprint.
-
Oropharyngeal Manifestations in Patients with HIV from Northeastern Romania.Medicina (Kaunas). 2025 May 6;61(5):855. doi: 10.3390/medicina61050855. Medicina (Kaunas). 2025. PMID: 40428813 Free PMC article.
References
-
- United Nations General Assembly Resolution No A/RES/70/266, Political Declaration on HIV and AIDS: On the Fast-Track to Accelerate the Fight against HIV and to End the AIDS Epidemic by 2030. 2016. [(accessed on 20 June 2021)]. pp. 1–26. Available online: https://www.unaids.org/sites/default/files/media_asset/2016-political-de....
-
- World Health Organization Guideline on When to Start Antiretroviral Therapy and on Pre-Exposure Prophylaxis for HIV, WHO. 2015. [(accessed on 19 June 2021)]. pp. 1–78. Available online: http://apps.who.int/iris/bitstream/handle/10665/186275/9789241509565_eng.... - PubMed
-
- UNAIDS Data 2020. [(accessed on 21 June 2021)]. Available online: https://www.unaids.org/sites/default/files/media_asset/2020_aids-data-bo....
-
- Aghokeng A.F., Monleau M., Eymard-Duvernay S., Dagnra A., Kania D., Ngo-Giang-Huong N., Toni T.D., Touré-Kane C., Truong L.X., Delaporte E., et al. Extraordinary heterogeneity of virological outcomes in patients receiving highly antiretroviral therapy and monitored with the World Health Organization public health approach in sub-saharan Africa and southeast Asia. Clin. Infect. Dis. 2014;58:99–109. doi: 10.1093/cid/cit627. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

