Characterization of Ternary CuNiCo Metallic Nanoparticles Produced by Hydrogen Reduction
- PMID: 34683598
- PMCID: PMC8541350
- DOI: 10.3390/ma14206006
Characterization of Ternary CuNiCo Metallic Nanoparticles Produced by Hydrogen Reduction
Abstract
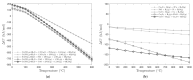
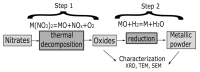
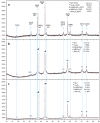
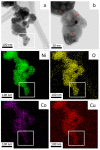
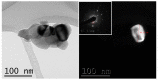
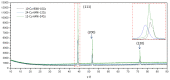
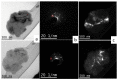
Different methods of producing nanostructured materials at the laboratory scale have been studied using a variety of physical and chemical techniques, though the challenge here is the homogeneous distribution of the elements which also depends on the precursor elements. This work thus focused on the micro-analytical characterization of Cu-Ni-Co metallic nanoparticles produced by an alternative chemical route aiming to produce solid solution nanoparticles. This method was based on two steps: the co-formation of oxides by nitrates' decomposition followed by their hydrogen reduction. Based on the initial composition of precursor nitrates, three homogeneous ternaries of the Ni, Cu and Co final alloy products were pre-established. Thus, the compositions in %wt of the synthesized alloy particles studied in this work are 24Cu-64Ni-12Co, 12Cu-64Ni-24Co and 10Cu-80Ni-10Co. Both precursor oxides and metallic powders were characterized by means of X-ray powder diffraction (XRD), scanning electron microscopy (SEM/EDS) and transmission electron microscopy (TEM). The results show that the synthesis procedure was successful since it produced a homogeneous material distributed in different particle sizes depending on the temperature applied in the reducing process. The final composition of the metallic product was consistent with what was theoretically expected. Resulting from reduction at the lower temperature of 300 °C, the main powder product consisted of particles with a spheroidal and eventually facetted morphology of 50 nm on average, which shared the same FCC crystal structure. Particles smaller than 100 nm in the Cu-Ni-Co alloy agglomerates were also observed. At a higher reduction temperature, the ternary powder developed robust particles of 1 micron in size, which are, in fact, the result of the coarsening of several nanoparticles.
Keywords: Cu–Ni–Co alloy; characterization; hydrogen reduction; nanoparticles.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Zach M., Hagglund C., Chakarov D., Kasemo B. Nanoscience and nanotechnology for advanced energy systems. Curr. Opin. Solid State Mater. Sci. 2006;10:132–143. doi: 10.1016/j.cossms.2007.04.004. - DOI
-
- Islam N., Miyazaki K. Nanotechnology innovation system: Understanding hidden dynamics of nanoscience fusion trajectories. Technol. Forecast. Soc. Chang. 2009;76:128–140. doi: 10.1016/j.techfore.2008.03.021. - DOI
-
- Mondal B., Basumallick A., Chattopadhyay P. Magnetic behavior of nanocrystalline Cu-Ni-Co alloys prepared by mechanical alloying and isothermal annealing. J. Alloys Compd. 2008;457:10–14. doi: 10.1016/j.jallcom.2007.02.139. - DOI
-
- Chai Z., Jiang C., Zhao Y., Wang C., Zhu K., Cai F. Microstructural characterization and corrosion behaviors of Ni-Cu-Co coatings electrodeposited in sulphate-citrate bath with additives. Surf. Coat. Technol. 2016;307:817–824. doi: 10.1016/j.surfcoat.2016.10.009. - DOI
-
- Pané S., Gómez E., Vallés E. Magnetoresistive granular Cu-Co-Ni coatings prepared by electrodeposition. J. Electroanal. Chem. 2006;596:87–94. doi: 10.1016/j.jelechem.2006.07.009. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

