Drug Penetration into the Central Nervous System: Pharmacokinetic Concepts and In Vitro Model Systems
- PMID: 34683835
- PMCID: PMC8538549
- DOI: 10.3390/pharmaceutics13101542
Drug Penetration into the Central Nervous System: Pharmacokinetic Concepts and In Vitro Model Systems
Abstract
Delivery of most drugs into the central nervous system (CNS) is restricted by the blood-brain barrier (BBB), which remains a significant bottleneck for development of novel CNS-targeted therapeutics or molecular tracers for neuroimaging. Consistent failure to reliably predict drug efficiency based on single measures for the rate or extent of brain penetration has led to the emergence of a more holistic framework that integrates data from various in vivo, in situ and in vitro assays to obtain a comprehensive description of drug delivery to and distribution within the brain. Coupled with ongoing development of suitable in vitro BBB models, this integrated approach promises to reduce the incidence of costly late-stage failures in CNS drug development, and could help to overcome some of the technical, economic and ethical issues associated with in vivo studies in animal models. Here, we provide an overview of BBB structure and function in vivo, and a summary of the pharmacokinetic parameters that can be used to determine and predict the rate and extent of drug penetration into the brain. We also review different in vitro models with regard to their inherent shortcomings and potential usefulness for development of fast-acting drugs or neurotracers labeled with short-lived radionuclides. In this regard, a special focus has been set on those systems that are sufficiently well established to be used in laboratories without significant bioengineering expertise.
Keywords: BBB permeability; co-culture model; dynamic BBB model; immobilized artificial membrane (IAM) chromatography; microfluidic BBB model; monolayer; neurotracer; parallel artificial membrane permeability assay (PAMPA); positron emission tomography (PET); static BBB model.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






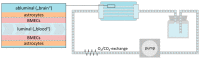

References
-
- Pardridge W.M., Triguero D., Yang J., Cancilla P.A. Comparison of in vitro and in vivo models of drug transcytosis through the blood-brain barrier. J. Pharmacol. Exp. Ther. 1990;253:884–891. - PubMed
-
- Bradbury M.W.B. The Concept of a Blood Brain Barrier. Wiley; Chichester, UK: 1979.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

