Discovery and Validation of a Compound to Target Ewing's Sarcoma
- PMID: 34683845
- PMCID: PMC8538197
- DOI: 10.3390/pharmaceutics13101553
Discovery and Validation of a Compound to Target Ewing's Sarcoma
Abstract
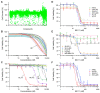
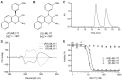
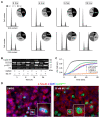
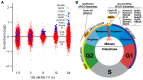
Ewing's sarcoma, characterized by pathognomonic t (11; 22) (q24; q12) and related chromosomal ETS family translocations, is a rare aggressive cancer of bone and soft tissue. Current protocols that include cytotoxic chemotherapeutic agents effectively treat localized disease; however, these aggressive therapies may result in treatment-related morbidities including second-site cancers in survivors. Moreover, the five-year survival rate in patients with relapsed, recurrent, or metastatic disease is less than 30%, despite intensive therapy with these cytotoxic agents. By using high-throughput phenotypic screening of small molecule libraries, we identified a previously uncharacterized compound (ML111) that inhibited in vitro proliferation of six established Ewing's sarcoma cell lines with nanomolar potency. Proteomic studies show that ML111 treatment induced prometaphase arrest followed by rapid caspase-dependent apoptotic cell death in Ewing's sarcoma cell lines. ML111, delivered via methoxypoly(ethylene glycol)-polycaprolactone copolymer nanoparticles, induced dose-dependent inhibition of Ewing's sarcoma tumor growth in a murine xenograft model and invoked prometaphase arrest in vivo, consistent with in vitro data. These results suggest that ML111 represents a promising new drug lead for further preclinical studies and is a potential clinical development for the treatment of Ewing's sarcoma.
Keywords: Ewing’s sarcoma; ML111; cancer; chemotherapy; drug discovery; high-throughput screening; nanoparticle drug delivery.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Bailey K., Cost C., Davis I., Glade-Bender J., Grohar P., Houghton P., Isakoff M., Stewart E., Laack N., Yustein J., et al. Emerging Novel Agents for Patients with Advanced Ewing Sarcoma: A Report from the Children’s Oncology Group (COG) New Agents for Ewing Sarcoma Task Force. F1000Research. 2019;8:493. doi: 10.12688/f1000research.18139.1. - DOI - PMC - PubMed
-
- Womer R.B., West D.C., Krailo M.D., Dickman P.S., Pawel B.R., Grier H.E., Marcus K., Sailer S., Healey J.H., Dormans J.P., et al. Randomized Controlled Trial of Interval-Compressed Chemotherapy for the Treatment of Localized Ewing Sarcoma: A Report from the Children’s Oncology Group. J. Clin. Oncol. 2012;30:4148–4154. doi: 10.1200/JCO.2011.41.5703. - DOI - PMC - PubMed
-
- Lessnick S.L., Braun B.S., Denny C.T., May W.A. Multiple Domains Mediate Transformation by the Ewing’s Sarcoma EWS/FLI-1 Fusion Gene. Oncogene. 1995;10:423–431. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

