Application of Nanomaterials as an Advanced Strategy for the Diagnosis, Prevention, and Treatment of Viral Diseases
- PMID: 34683863
- PMCID: PMC8540357
- DOI: 10.3390/pharmaceutics13101570
Application of Nanomaterials as an Advanced Strategy for the Diagnosis, Prevention, and Treatment of Viral Diseases
Abstract
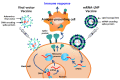
The coronavirus disease (COVID-19) pandemic poses serious global health concerns with the continued emergence of new variants. The periodic outbreak of novel emerging and re-emerging infectious pathogens has elevated concerns and challenges for the future. To develop mitigation strategies against infectious diseases, nano-based approaches are being increasingly applied in diagnostic systems, prophylactic vaccines, and therapeutics. This review presents the properties of various nanoplatforms and discusses their role in the development of sensors, vectors, delivery agents, intrinsic immunostimulants, and viral inhibitors. Advanced nanomedical applications for infectious diseases have been highlighted. Moreover, physicochemical properties that confer physiological advantages and contribute to the control and inhibition of infectious diseases have been discussed. Safety concerns limit the commercial production and clinical use of these technologies in humans; however, overcoming these limitations may enable the use of nanomaterials to resolve current infection control issues via application of nanomaterials as a platform for the diagnosis, prevention, and treatment of viral diseases.
Keywords: diagnosis; nanomaterials; therapeutics; vaccines; viral diseases.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Advanced Nanomaterials for Preparedness Against (Re-)Emerging Viral Diseases.Adv Mater. 2021 Nov;33(47):e2005927. doi: 10.1002/adma.202005927. Epub 2021 Feb 15. Adv Mater. 2021. PMID: 33586180 Review.
-
Advancements in protein nanoparticle vaccine platforms to combat infectious disease.Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2021 May;13(3):e1681. doi: 10.1002/wnan.1681. Epub 2020 Nov 8. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2021. PMID: 33164326 Free PMC article. Review.
-
Applications of nanomaterials in COVID-19 pandemic.Rare Metals. 2022;41(1):1-13. doi: 10.1007/s12598-021-01789-y. Epub 2021 Sep 15. Rare Metals. 2022. PMID: 34539132 Free PMC article. Review.
-
The role of nanotechnology in current COVID-19 outbreak.Heliyon. 2021 Apr;7(4):e06841. doi: 10.1016/j.heliyon.2021.e06841. Epub 2021 Apr 15. Heliyon. 2021. PMID: 33880422 Free PMC article. Review.
-
Role of different types of nanomaterials against diagnosis, prevention and therapy of COVID-19.Sustain Cities Soc. 2021 Sep;72:103046. doi: 10.1016/j.scs.2021.103046. Epub 2021 May 25. Sustain Cities Soc. 2021. PMID: 34055576 Free PMC article. Review.
Cited by
-
Colorimetry-Based Phosphate Measurement for Polymerase Elongation.Biomed Res Int. 2023 Jan 23;2023:8296847. doi: 10.1155/2023/8296847. eCollection 2023. Biomed Res Int. 2023. PMID: 36726843 Free PMC article.
-
Nanomaterials-Based Wound Dressing for Advanced Management of Infected Wound.Antibiotics (Basel). 2023 Feb 8;12(2):351. doi: 10.3390/antibiotics12020351. Antibiotics (Basel). 2023. PMID: 36830262 Free PMC article. Review.
-
Nanoparticles in clinical trials of COVID-19: An update.Int J Surg. 2022 Aug;104:106818. doi: 10.1016/j.ijsu.2022.106818. Epub 2022 Aug 8. Int J Surg. 2022. PMID: 35953020 Free PMC article. Review.
-
Use of Graphene and Its Derivatives for the Detection of Dengue Virus.Biosensors (Basel). 2023 Mar 6;13(3):349. doi: 10.3390/bios13030349. Biosensors (Basel). 2023. PMID: 36979561 Free PMC article. Review.
-
Emerging Applications of Biomedical Science in Pandemic Prevention and Control: A Review.Cureus. 2023 Aug 24;15(8):e44075. doi: 10.7759/cureus.44075. eCollection 2023 Aug. Cureus. 2023. PMID: 37750154 Free PMC article. Review.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

