Human Skin Permeation Enhancement Using PLGA Nanoparticles Is Mediated by Local pH Changes
- PMID: 34683901
- PMCID: PMC8538358
- DOI: 10.3390/pharmaceutics13101608
Human Skin Permeation Enhancement Using PLGA Nanoparticles Is Mediated by Local pH Changes
Abstract
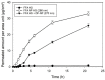
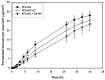
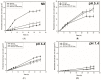
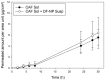
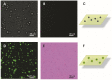
The steady improvement and optimization of transdermal permeation is a constant and challenging pharmaceutical task. In this study the influence of poly(lactide-co-glycolide) (PLGA) nanoparticles on the dermal permeation of the anti-inflammatory drug flufenamic acid (FFA) was investigated. For this aim, different vehicles under non-buffered and buffered conditions and different skin models (human heat separated epidermis and reconstructed human epidermis equivalents) were tested. Permeation experiments were performed using static Franz diffusion cells under infinite dosing conditions. Already the presence of drug-free nanoparticles increased drug permeation across the skin. Drug permeation was even enhanced when applying drug-loaded nanoparticles. In contrast, buffered vehicles with different pH values (pH 5.4-7.4) revealed the influence of the pH on the permeation of FFA. The change of the surrounding pH of the biodegradable nanoparticulate system was demonstrated and visualized using pH-sensitive fluorescent probes. While a potential contribution of hair follicles could be ruled out, our data suggest that the enhanced permeation of FFA through human skin in the presence of PLGA nanoparticles is mediated by a locally decreased pH during hydrolytic degradation of this polymer. This hypothesis is supported by the observation that skin permeation of the weak base caffeine was not affected.
Keywords: PLGA; human skin; pH effects; penetration; permeation; polymer nanoparticles.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Rodríguez Cruz I., Domínguez-Delgado C., Escobar-Chávez J., López-Cervantes M., Díaz-Torres R. Current Technologies to Increase the Transdermal Delivery of Drugs. Volume 2. Bentham Science Publishers; Sharjah, United Arab Emirates: 2016. Physical Penetration Enhancers: An Overview; pp. 3–34.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

