Enhanced Detection of Desmoplasia by Targeted Delivery of Iron Oxide Nanoparticles to the Tumour-Specific Extracellular Matrix
- PMID: 34683956
- PMCID: PMC8539756
- DOI: 10.3390/pharmaceutics13101663
Enhanced Detection of Desmoplasia by Targeted Delivery of Iron Oxide Nanoparticles to the Tumour-Specific Extracellular Matrix
Abstract
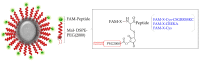
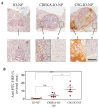
Diagnostic imaging of aggressive cancer with a high stroma content may benefit from the use of imaging contrast agents targeted with peptides that have high binding affinity to the extracellular matrix (ECM). In this study, we report the use of superparamagnetic iron-oxide nanoparticles (IO-NP) conjugated to a nonapeptide, CSGRRSSKC (CSG), which specifically binds to the laminin-nidogen-1 complex in tumours. We show that CSG-IO-NP accumulate in tumours, predominantly in the tumour ECM, following intravenous injection into a murine model of pancreatic neuroendocrine tumour (PNET). In contrast, a control untargeted IO-NP consistently show poor tumour uptake, and IO-NP conjugated to a pentapeptide. CREKA that bind fibrin clots in blood vessels show restricted uptake in the angiogenic vessels of the tumours. CSG-IO-NP show three-fold higher intratumoral accumulation compared to CREKA-IO-NP. Magnetic resonance imaging (MRI) T2-weighted scans and T2 relaxation times indicate significant uptake of CSG-IO-NP irrespective of tumour size, whereas the uptake of CREKA-IO-NP is only consistent in small tumours of less than 3 mm in diameter. Larger tumours with significantly reduced tumour blood vessels show a lack of CREKA-IO-NP uptake. Our data suggest CSG-IO-NP are particularly useful for detecting stroma in early and advanced solid tumours.
Keywords: CSG; extracellular matrix; magnetic resonance imaging; nanoparticles; tumour targeting.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures





References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

