Towards a Better Understanding of Verapamil Release from Kollicoat SR:IR Coated Pellets Using Non-Invasive Analytical Tools
- PMID: 34684015
- PMCID: PMC8541620
- DOI: 10.3390/pharmaceutics13101723
Towards a Better Understanding of Verapamil Release from Kollicoat SR:IR Coated Pellets Using Non-Invasive Analytical Tools
Abstract
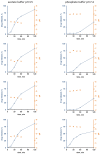
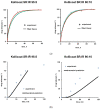
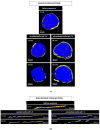
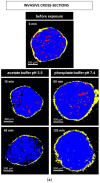
The aim of this study was to gain deeper insight into the mass transport mechanisms controlling drug release from polymer-coated pellets using non-invasive analytical tools. Pellet starter cores loaded with verapamil HCl (10% loading, 45% lactose, 45% microcrystalline cellulose) were prepared by extrusion/spheronization and coated with 5% Kollicoat SR:IR 95:5 or 10% Kollicoat SR:IR 90:10. Drug release was measured from ensembles of pellets as well as from single pellets upon exposure to acetate buffer pH = 3.5 and phosphate buffer pH = 7.4. The swelling of single pellets was observed by optical microscopy, while dynamic changes in the pH in the pellet cores were monitored by fluorescence spectroscopy. Also, mathematical modeling using a mechanistically realistic theory as well as SEM and Raman imaging were applied to elucidate whether drug release mainly occurs by diffusion through the intact film coatings or whether crack formation in the film coatings plays a role. Interestingly, fluorescence spectroscopy revealed that the pH within the pellet cores substantially differed upon exposure to acetate buffer pH = 3.5 and phosphate buffer pH = 7.4, resulting in significant differences in drug solubility (verapamil being a weak base) and faster drug release at lower pH: from ensembles of pellets and single pellets. The monitoring of drug release from and the swelling of single pellets indicated that crack formation in the film coatings likely plays a major role, irrespective of the Kollicoat SR:IR ratio/coating level. This was confirmed by mathematical modeling, SEM and Raman imaging. Importantly, the latter technique allowed also for non-invasive measurements, reducing the risk of artifact creation associated with sample cutting with a scalpel.
Keywords: coated pellet; controlled release; drug release mechanisms; film coating; non-invasive analytical tools; polyvinyl acetate; weakly basic drug.
Conflict of interest statement
The authors declare no conflict of interest. The Bayer AG company had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
Figures






Similar articles
-
Development of a novel osmotically driven drug delivery system for weakly basic drugs.Eur J Pharm Biopharm. 2008 Jun;69(2):667-74. doi: 10.1016/j.ejpb.2007.12.017. Epub 2007 Dec 28. Eur J Pharm Biopharm. 2008. PMID: 18226884
-
Polyvinyl acetate-based film coatings.Int J Pharm. 2013 Dec 5;457(2):470-9. doi: 10.1016/j.ijpharm.2013.08.077. Epub 2013 Sep 25. Int J Pharm. 2013. PMID: 24076229 Review.
-
Influence of the components of Kollicoat SR film on mechanical properties of floating pellets from the point of view of tableting.Pharmazie. 2008 Oct;63(10):731-5. Pharmazie. 2008. PMID: 18972835
-
Importance of air bubbles in the core of coated pellets: Synchrotron X-ray microtomography allows for new insights.J Control Release. 2016 Sep 10;237:125-37. doi: 10.1016/j.jconrel.2016.06.041. Epub 2016 Jul 1. J Control Release. 2016. PMID: 27374626
-
Application of terahertz pulsed imaging to analyse film coating characteristics of sustained-release coated pellets.Int J Pharm. 2013 Dec 5;457(2):521-6. doi: 10.1016/j.ijpharm.2013.05.039. Epub 2013 May 27. Int J Pharm. 2013. PMID: 23721891 Review.
Cited by
-
Design and Optimization of a Nanoparticulate Pore Former as a Multifunctional Coating Excipient for pH Transition-Independent Controlled Release of Weakly Basic Drugs for Oral Drug Delivery.Pharmaceutics. 2023 Feb 7;15(2):547. doi: 10.3390/pharmaceutics15020547. Pharmaceutics. 2023. PMID: 36839869 Free PMC article.
-
Investigating in-vitro functionality and in-vivo taste assessment of eco-friendly Tadalafil Pastilles.Heliyon. 2024 Apr 16;10(8):e29543. doi: 10.1016/j.heliyon.2024.e29543. eCollection 2024 Apr 30. Heliyon. 2024. PMID: 38660288 Free PMC article.
-
Drug-Release Mechanisms Elucidated by Imaging Techniques: Visualizing the Invisible!Pharmaceutics. 2022 May 30;14(6):1165. doi: 10.3390/pharmaceutics14061165. Pharmaceutics. 2022. PMID: 35745737 Free PMC article.
-
Wurster Technology-Assisted Step-by-Step Engineering of Multi-layered Pellets (Sprinkles): Microscopy, Micro-CT, and e-Tongue-Based Analysis.AAPS PharmSciTech. 2024 Feb 29;25(3):50. doi: 10.1208/s12249-024-02773-2. AAPS PharmSciTech. 2024. PMID: 38424241
References
-
- Ghebre-Sellassie I. Multiparticulate Oral Drug Delivery. Marcel Dekker; New York, NY, USA: 1997.
-
- Brunner M., Greinwald R., Kletter K., Kvaternik H., Corrado M.E., Eichler H.G., Müller M. Gastrointestinal Transit and Release of 5-Aminosalicylic Acid from 153 Sm-Labelled Mesalazine Pellets vs. Tablets in Male Healthy Volunteers. Aliment. Pharmacol. Ther. 2003;17:1163. doi: 10.1046/j.1365-2036.2003.01564.x. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

