Design of a Platelet-Mediated Delivery System for Drug-Incorporated Nanospheres to Enhance Anti-Tumor Therapeutic Effect
- PMID: 34684017
- PMCID: PMC8540062
- DOI: 10.3390/pharmaceutics13101724
Design of a Platelet-Mediated Delivery System for Drug-Incorporated Nanospheres to Enhance Anti-Tumor Therapeutic Effect
Abstract
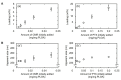
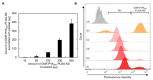
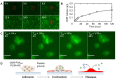
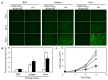
The objective of this study is to construct a platelet-mediated delivery system for drug-incorporated nanospheres. Nanospheres of poly(lactic-co-glycolic acid) (PLGA-NS) with different sizes and surface properties were prepared by changing the preparation parameters, such as the type of polymer surfactant, the concentration of polymer surfactant and PLGA, and the stirring rate. When incubated with platelets, PLGA-NS prepared with poly(vinyl alcohol) suppressed the platelet activation. Scanning electron microscopic and flow cytometry examinations revealed that platelets associated with PLGA-NS (platelet hybrids, PH) had a similar appearance and biological properties to those of the original platelets. In addition, the PH with PLGA-NS specifically adhered onto the substrate pre-coated with fibrin to a significantly great extent compared with PLGA-NS alone. When applied in an in vitro model of tumor tissue which was composed of an upper chamber pre-coated with fibrin and a lower chamber culturing tumor cells, the PH with PLGA-NS incorporating an anti-tumor drug were delivered to the tumor cells through the specific adhesion onto the upper chamber and, consequently, drug release from the upper chamber took place, resulting in the growth suppression of tumor cells. It is concluded that the drug delivery system based on PH is promising for tumor treatment.
Keywords: drug delivery system; nanopsheres; platelets; poly(lactic-co-glycolic acid); tumor.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

