Optimization and Development of Selective Histone Deacetylase Inhibitor (MPT0B291)-Loaded Albumin Nanoparticles for Anticancer Therapy
- PMID: 34684020
- PMCID: PMC8541575
- DOI: 10.3390/pharmaceutics13101728
Optimization and Development of Selective Histone Deacetylase Inhibitor (MPT0B291)-Loaded Albumin Nanoparticles for Anticancer Therapy
Abstract
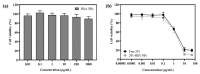
Histone deacetylase (HDAC) inhibitors have emerged as a new class of antitumor agent for various types of tumors. MPT0B291, a novel selective inhibitor of HDAC6, demonstrated significant antiproliferative activity in various human cancer cell types. However, MPT0B291 has very low water solubility, which limits its clinical use for cancer therapy. In the current study, MPT0B291 was encapsulated in human serum albumin (HSA), and its anticancer activities were investigated. Nanoparticles (NPs) were prepared using two-stage emulsification resulting in 100~200-nm NPs with a fine size distribution (polydispersity index of <0.3). The in vitro drug release profiles of MPT0B291-loaded HSA NPs presented sustained-release properties. The cytotoxic effect on MIA PaCa-2 human pancreatic carcinoma cells was found to be similar to MPT0B291-loaded HSA NPs and the free-drug group. The albumin-based formulation provided a higher maximum tolerated dose than that of a drug solution with reduced toxicity toward normal cells. Furthermore, in vivo pharmacokinetic studies demonstrated an effective increase (5~8-fold) in the bioavailability of NPs containing MPT0B291 loaded in HSA compared to the free-drug solution with an extended circulation time (t1/2) leading to significantly enhanced efficacy of anticancer treatment.
Keywords: MPT0B291; albumin nanoparticle; high-pressure homogenizer; histone deacetylase.
Conflict of interest statement
The authors declare no conflict of interest. The funder had no role in the design of the study, in the collection, analyses or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
Figures









References
-
- Fraga M.F., Ballestar E., Villar-Garea A., Boix-Chornet M., Espada J., Schotta G., Bonaldi T., Haydon C., Ropero S., Petrie K. Loss of acetylation at lys16 and trimethylation at lys20 of histone h4 is a common hallmark of human cancer. Nat. Genet. 2005;37:391–400. doi: 10.1038/ng1531. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

