Infant Formula Based on Milk Fat Affects Immune Development in Both Normal Birthweight and Fetal Growth Restricted Neonatal Piglets
- PMID: 34684311
- PMCID: PMC8539276
- DOI: 10.3390/nu13103310
Infant Formula Based on Milk Fat Affects Immune Development in Both Normal Birthweight and Fetal Growth Restricted Neonatal Piglets
Abstract
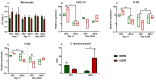
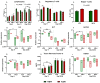
Infant formulas offer an alternative to breast milk for both normal birth weight (NBW) and immunocompromised intrauterine growth restricted (IUGR) infants. Although the lipid fraction in formulas is often derived from vegetable oils, it is unclear if this alters immunological outcomes relative to milk fats or whether these effects differ between IUGR and NBW infants. We hypothesized that replacing vegetable oil with bovine milk fat in infant formula would improve immune development in IUGR and NBW neonates. Two-day old piglets were selected (NBW, n = 18, IUGR, n = 18) and each group of animals were fed formula based on either vegetable oil (VEG) or bovine milk fat (MILK). Animals were reared until day 23/24 and systemic immune parameters were evaluated. Milk-fat feeding decreased blood neutrophil counts and improved neutrophil function while transiently reducing leucocytes' expression of genes related to adaptive and innate immunity as well as energy metabolism, following in vitro stimulation by live Staphylococcus epidermidis (whole blood, 2 h). However, there were only a few interactions between milk-fat type and birthweight status. Thus, piglets fed milk-fat-based formula had improved neutrophil maturation and suppressed pro-inflammatory responses, compared to those fed vegetable-oil-based formula.
Keywords: immune development; infant; infant formula; intrauterine growth restriction; milk fat; neonate.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Brain lipidomics and neurodevelopmental outcomes in intrauterine growth restricted piglets fed dairy or vegetable fat diets.Sci Rep. 2022 Feb 28;12(1):3303. doi: 10.1038/s41598-022-07133-3. Sci Rep. 2022. PMID: 35228576 Free PMC article.
-
Effects of high nutrient intake on the growth performance, intestinal morphology and immune function of neonatal intra-uterine growth-retarded pigs.Br J Nutr. 2013 Nov;110(10):1819-27. doi: 10.1017/S0007114513001232. Epub 2013 Apr 19. Br J Nutr. 2013. PMID: 23596997
-
A mixture of milk and vegetable lipids in infant formula changes gut digestion, mucosal immunity and microbiota composition in neonatal piglets.Eur J Nutr. 2018 Mar;57(2):463-476. doi: 10.1007/s00394-016-1329-3. Epub 2016 Oct 15. Eur J Nutr. 2018. PMID: 27744547
-
The Role of Lactoferrin in Gastrointestinal and Immune Development and Function: A Preclinical Perspective.J Pediatr. 2016 Jun;173 Suppl:S16-28. doi: 10.1016/j.jpeds.2016.02.072. J Pediatr. 2016. PMID: 27234407 Review.
-
Biological roles of milk osteopontin.Curr Opin Clin Nutr Metab Care. 2016 May;19(3):214-9. Curr Opin Clin Nutr Metab Care. 2016. PMID: 27504516 Review.
Cited by
-
Enteral plasma feeding improves gut function and immunity in piglets after birth asphyxia.Pediatr Res. 2025 Feb;97(2):774-784. doi: 10.1038/s41390-024-03376-0. Epub 2024 Jul 21. Pediatr Res. 2025. PMID: 39034356 Free PMC article.
-
Developmental Changes of Immunity and Different Responses to Weaning Stress of Chinese Indigenous Piglets and Duroc Piglets during Suckling and Weaning Periods.Int J Mol Sci. 2022 Dec 12;23(24):15781. doi: 10.3390/ijms232415781. Int J Mol Sci. 2022. PMID: 36555421 Free PMC article.
-
Brain lipidomics and neurodevelopmental outcomes in intrauterine growth restricted piglets fed dairy or vegetable fat diets.Sci Rep. 2022 Feb 28;12(1):3303. doi: 10.1038/s41598-022-07133-3. Sci Rep. 2022. PMID: 35228576 Free PMC article.
-
A Comparison of Haematological and Biochemical Profiles between Intrauterine Growth Restriction and Normal Piglets at 72 Hours Postpartum.Animals (Basel). 2023 Nov 16;13(22):3540. doi: 10.3390/ani13223540. Animals (Basel). 2023. PMID: 38003158 Free PMC article.
References
-
- El Manouni el Hassani S., Berkhout D.J.C., Niemarkt H.J., Mann S., de Boode W.P., Cossey V., Hulzebos C.V., van Kaam A.H., Kramer B.W., van Lingen R.A., et al. Risk Factors for Late-Onset Sepsis in Preterm Infants: A Multicenter Case-Control Study. Neonatology. 2019;116:42–51. doi: 10.1159/000497781. - DOI - PMC - PubMed
-
- Watts T., Roberts I. Haematological abnormalities in the growth-restricted infant. Semin. Neonatol. 1999;4:41–54. doi: 10.1016/S1084-2756(99)80006-2. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

