Berberine Phospholipid Is an Effective Insulin Sensitizer and Improves Metabolic and Hormonal Disorders in Women with Polycystic Ovary Syndrome: A One-Group Pretest-Post-Test Explanatory Study
- PMID: 34684666
- PMCID: PMC8538182
- DOI: 10.3390/nu13103665
Berberine Phospholipid Is an Effective Insulin Sensitizer and Improves Metabolic and Hormonal Disorders in Women with Polycystic Ovary Syndrome: A One-Group Pretest-Post-Test Explanatory Study
Abstract
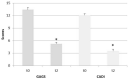
Polycystic Ovary Syndrome (PCOS) is the most frequent endocrine disease in females of reproductive age and is characterized by multifactorial unhealthy conditions related to hormonal unbalance and also to dysmetabolism and inflammation. Recently, increasing evidence has shown that natural plant-based products may play a role in PCOS management. The aim of this one-group pretest-post-test explanatory study was to evaluate, in normal-overweight PCOS women with normal menses, the effectiveness of berberine on: Insulin resistance (IR) by Homeostasis Model Assessment (HOMA); Inflammation by C-Reactive Protein (CRP), Tumor Necrosis Factor α (TNF-α); Lipid metabolism; Sex hormone profile and symptoms correlated to hyperandrogenism, such as acne, by Global Acne Grading System (GAGS) and Cardiff Acne Disability Index (CADI); Body composition by DXA. Finally, adverse effects were assessed by liver and kidney functions and creatine phosphokinase (CPK). All these parameters were collected at baseline and 60 days after supplementation with a new bioavailable and safe berberine formulation. Twelve females (aged 26.6 ± 4.9, BMI 25.3 ± 3.6) were supplied for 60 days with two tablets/day (550 mg/table) of the bioavailable berberine. Results showed a statistically significant decrease in HOMA, CRP, TNF-α, Triglycerides, testosterone, Body Mass Index (BMI), Visceral Adipose Tissue (VAT), fat mass, GAGS and CADI scores, and a statistically significant increase in sex hormone-binding globulin (SHBG). Liver and kidney functions and CPK are not statistically significantly different. Therefore, berberine can represent a safe novel dietary supplement, helpful in treatment strategy for PCOS.
Keywords: acne symptoms; berberine; homeostasis model assessment; inflammation; phytosome; polycystic ovary syndrome; visceral adipose tissue.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
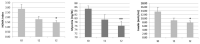

References
-
- Rondanelli M., Perna S., Faliva M., Monteferrario F., Repaci E., Allieri F. Focus on metabolic and nutritional correlates of polycystic ovary syndrome and update on nutritional management of these critical phenomena. Arch. Gynecol. Obstet. 2014;290:1079–1092. doi: 10.1007/s00404-014-3433-z. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

