Porous 3D Scaffolds Enhance MSC Vitality and Reduce Osteoclast Activity
- PMID: 34684837
- PMCID: PMC8541337
- DOI: 10.3390/molecules26206258
Porous 3D Scaffolds Enhance MSC Vitality and Reduce Osteoclast Activity
Abstract
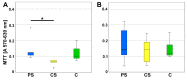
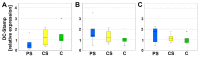
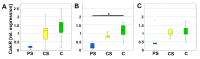
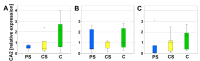
In the context of an aging population, unhealthy Western lifestyle, and the lack of an optimal surgical treatment, deep osteochondral defects pose a great challenge for the public health system. Biodegradable, biomimetic scaffolds seem to be a promising solution. In this study we investigated the biocompatibility of porous poly-((D,L)-lactide-ε-caprolactone)dimethacrylate (LCM) scaffolds in contrast to compact LCM scaffolds and blank cell culture plastic. Thus, morphology, cytotoxicity and metabolic activity of human mesenchymal stromal cells (MSC) seeded directly on the materials were analyzed after three and six days of culturing. Further, osteoclastogenesis and osteoclastic activity were assessed using reverse-transcriptase real-time PCR of osteoclast-specific genes, EIA and morphologic aspects after four, eight, and twelve days. LCM scaffolds did not display cytotoxic effects on MSC. After three days, metabolic activity of MSC was enhanced on 3D porous scaffolds (PS) compared to 2D compact scaffolds (CS). Osteoclast activity seemed to be reduced at PS compared to cell culture plastic at all time points, while no differences in osteoclastogenesis were detectable between the materials. These results indicate a good cytocompatibility of LCM scaffolds. Interestingly, porous 3D structure induced higher metabolic activity of MSC as well as reduced osteoclast activity.
Keywords: 3D-printing; bone; cartilage; implant; mRNA expression; mesenchymal stromal cells; osteoclasts; poly-lactide-caprolactone; polymer; tartrate-resistant acid phosphatase.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures







References
-
- Sartori M., Pagani S., Ferrari A., Costa V., Carina V., Figallo E., Maltarello M.C., Martini L., Fini M., Giavaresi G. A new bi-layered scaffold for osteochondral tissue regeneration: In Vitro and in vivo preclinical investigations. Mater. Sci. Eng. C Mater. Biol. Appl. 2017;70:101–111. doi: 10.1016/j.msec.2016.08.027. - DOI - PubMed
-
- Kampleitner C., Changi K., Felfel R.M., Scotchford C.A., Sottile V., Kluger R., Hoffmann O., Grant D.M., Epstein M.M. Preclinical biological and physicochemical evaluation of two-photon engineered 3D biomimetic copolymer scaffolds for bone healing. Biomater. Sci. 2020;8:1683–1694. doi: 10.1039/C9BM01827A. - DOI - PubMed
-
- Duan P., Pan Z., Cao L., Gao J., Yao H., Liu X., Guo R., Liang X., Dong J., Ding J. Restoration of osteochondral defects by implanting bilayered poly(lactide-co-glycolide) porous scaffolds in rabbit joints for 12 and 24 weeks. J. Orthop. Transl. 2019;19:68–80. doi: 10.1016/j.jot.2019.04.006. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

