d-Allulose Ameliorates Skeletal Muscle Insulin Resistance in High-Fat Diet-Fed Rats
- PMID: 34684891
- PMCID: PMC8539500
- DOI: 10.3390/molecules26206310
d-Allulose Ameliorates Skeletal Muscle Insulin Resistance in High-Fat Diet-Fed Rats
Abstract
Background: d-Allulose is a rare sugar with antiobesity and antidiabetic activities. However, its direct effect on insulin sensitivity and the underlying mechanism involved are unknown.
Objective: This study aimed to investigate the effect of d-allulose on high-fat diet (HFD)-induced insulin resistance using the hyperinsulinemic-euglycemic (HE)-clamp method and intramuscular signaling analysis.
Methods: Wistar rats were randomly divided into three dietary groups: chow diet, HFD with 5% cellulose (HFC), and HFD with 5% d-allulose (HFA). After four weeks of feeding, the insulin tolerance test (ITT), intraperitoneal glucose tolerance test (IPGTT), and HE-clamp study were performed. The levels of plasma leptin, adiponectin, and tumor necrosis factor (TNF)-α were measured using the enzyme-linked immunosorbent assay. We analyzed the levels of cell signaling pathway components in the skeletal muscle using Western blotting.
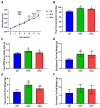
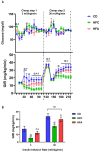
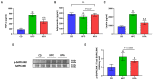
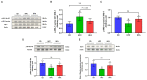
Results: d-allulose alleviated the increase in HFD-induced body weight and visceral fat and reduced the area under the curve as per ITT and IPGTT. d-Allulose increased the glucose infusion rate in the two-step HE-clamp test. Consistently, the insulin-induced phosphorylation of serine 307 in the insulin receptor substrate-1 and Akt and expression of glucose transporter 4 (Glut-4) in the muscle were higher in the HFA group than HFC group. Furthermore, d-allulose decreased plasma TNF-α concentration and insulin-induced phosphorylation of stress-activated protein kinase/Jun N-terminal kinase in the muscle and inhibited adiponectin secretion in HFD-fed rats.
Conclusions: d-allulose improved HFD-induced insulin resistance in Wistar rats. The reduction of the proinflammatory cytokine production, amelioration of adiponectin secretion, and increase in insulin signaling and Glut-4 expression in the muscle contributed to this effect.
Keywords: d-allulose; glucose uptake; hyperinsulinemic–euglycemic clamp; inflammation; insulin resistance; skeletal muscle; white adipose tissue.
Conflict of interest statement
This study was partly funded by Matsutani Chemical Industry Co., Ltd. (Itami, Japan). T.Y. and T.I. are employees of the company. The funders had no role in the design of the study; in the collection, analysis, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures

References
-
- Grice B.A., Barton K.J., Covert J.D., Kreilach A.M., Tackett L., Brozinick J.T., Elmendorf J.S. Excess membrane cholesterol is an early contributing reversible aspect of skeletal muscle insulin resistance in C57BL/6NJ mice fed a Western-style high-fat diet. Am. J. Physiol. Endocrinol. Metab. 2019;317:E362–E373. doi: 10.1152/ajpendo.00396.2018. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

