Assessment of Nanopollution from Commercial Products in Water Environments
- PMID: 34684978
- PMCID: PMC8539925
- DOI: 10.3390/nano11102537
Assessment of Nanopollution from Commercial Products in Water Environments
Abstract
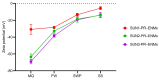
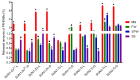
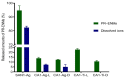
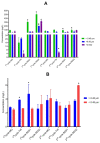
The use of nano-enabled products (NEPs) can release engineered nanomaterials (ENMs) into water resources, and the increasing commercialisation of NEPs raises the environmental exposure potential. The current study investigated the release of ENMs and their characteristics from six commercial products (sunscreens, body creams, sanitiser, and socks) containing nTiO2, nAg, and nZnO. ENMs were released in aqueous media from all investigated NEPs and were associated with ions (Ag+ and Zn2+) and coating agents (Si and Al). NEPs generally released elongated (7-9 × 66-70 nm) and angular (21-80 × 25-79 nm) nTiO2, near-spherical (12-49 nm) and angular nAg (21-76 × 29-77 nm), and angular nZnO (32-36 × 32-40 nm). NEPs released varying ENMs' total concentrations (ca 0.4-95%) of total Ti, Ag, Ag+, Zn, and Zn2+ relative to the initial amount of ENMs added in NEPs, influenced by the nature of the product and recipient water quality. The findings confirmed the use of the examined NEPs as sources of nanopollution in water resources, and the physicochemical properties of the nanopollutants were determined. Exposure assessment data from real-life sources are highly valuable for enriching the robust environmental risk assessment of nanotechnology.
Keywords: aquatic environments; engineered nanomaterials; nano-enabled products; nanopollution; physicochemical properties.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Foss Hansen S., Heggelund L.R., Revilla Besora P., Mackevica A., Boldrin A., Baun A., Hansen S.F., Heggelund L.R., Revilla Besora P., Mackevica A., et al. Nanoproducts—What is actually available to European consumers? Environ. Sci. Nano. 2016;3:169–180. doi: 10.1039/C5EN00182J. - DOI
-
- BCC Research Global Nanotechnology Market (by Component and Applications), Funding & Investment, Patent Analysis and 27 Companies Profile & Recent Developments—Forecast to 2024. [(accessed on 7 October 2020)]. Available online: https://www.bccresearch.com/market-research/nanotechnology.
-
- Vance M.E., Kuiken T., Vejerano E.P., McGinnis S.P., Hochella M.F., Rejeski D., Hull M.S., Hull M.S., Hull D.R., Rejeski D., et al. Nanotechnology in the real world: Redeveloping the nanomaterial consumer products inventory. Beilstein J. Nanotechnol. 2015;6:1769–1780. doi: 10.3762/bjnano.6.181. - DOI - PMC - PubMed
-
- PEN Inventory Finds Increase in Consumer Products Containing Nanoscale Materials. News Archive. Nanotechnology Project. [(accessed on 4 June 2019)]. Available online: http://www.nanotechproject.org/news/archive/9242/
-
- The Nanodatabase The Nanodatabase is developed by the DTU Environment, the Danish Ecological Council and Danish Consumer Council. [(accessed on 21 July 2017)]. Available online: http://nanodb.dk/en/about-us/
Grants and funding
LinkOut - more resources
Full Text Sources

