Highly Stable Pickering Emulsions with Xylan Hydrate Nanocrystals
- PMID: 34684997
- PMCID: PMC8537821
- DOI: 10.3390/nano11102558
Highly Stable Pickering Emulsions with Xylan Hydrate Nanocrystals
Abstract
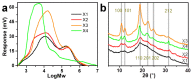
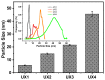
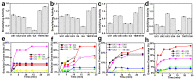
Xylan is a highly abundant plant-based biopolymer. Original xylans in plants are in an amorphous state, but deacetylated and low-branched xylan can form a crystalline structure with water molecules. The utilizations of xylan have been limited to bulk applications either with inconsistency and uncertainty or with extensive chemical derivatization due to the insufficient studies on its crystallization. The applications of xylan could be greatly broadened in advanced green materials if xylan crystals are effectively utilized. In this paper, we show a completely green production of nano-sized xylan crystals and propose their application in forming Pickering emulsions. The branches of xylan were regulated during the separation step to controllably induce the formation of xylan hydrate crystals. Xylan hydrate nanocrystals (XNCs) with a uniform size were successfully produced solely by a mild ultrasonic treatment. XNCs can be adsorbed onto oil-water interfaces at a high density to form highly stable Pickering emulsions. The emulsifying properties of XNCs were comparable to some synthetic emulsifiers and better than some other common biopolymer nanocrystals, demonstrating that XNCs have great potential in industrial emulsification.
Keywords: Pickering emulsion; hemicellulose; nanocrystal; xylan.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Ren J.-L., Sun R.-C. Hemicelluloses. In: Sun R.-C., editor. Cereal Straw as a Resource for Sustainable Biomaterials and Biofuels. Elsevier; Amsterdam, The Netherlands: 2010. pp. 73–130. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

