miR-21-3p/IL-22 Axes Are Major Drivers of Psoriasis Pathogenesis by Modulating Keratinocytes Proliferation-Survival Balance and Inflammatory Response
- PMID: 34685526
- PMCID: PMC8534095
- DOI: 10.3390/cells10102547
miR-21-3p/IL-22 Axes Are Major Drivers of Psoriasis Pathogenesis by Modulating Keratinocytes Proliferation-Survival Balance and Inflammatory Response
Abstract
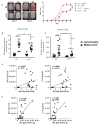
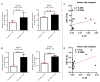
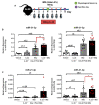
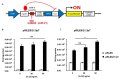
Psoriasis is a chronic inflammatory skin disease that is mediated by complex crosstalk between immune cells and keratinocytes (KCs). Emerging studies have showed a specific psoriatic microRNAs signature, in which miR-21 is one of the most upregulated and dynamic miRNAs. In this study, we focused our investigations on the passenger miR-21-3p strand, which is poorly studied in skin and in psoriasis pathogenesis. Here, we showed the upregulation of miR-21-3p in an IMQ-induced psoriasiform mouse model. This upregulation was correlated with IL-22 expression and functionality, both in vitro and in vivo, and it occurred via STAT3 and NF-κB signaling. We identified a network of differentially expressed genes involved in abnormal proliferation control and immune regulatory genes implicated in the molecular pathogenesis of psoriasis in response to miR-21-3p overexpression in KCs. These results were confirmed by functional assays that validated the proliferative potential of miR-21-3p. All these findings highlight the importance of miR-21-3p, an underestimated miRNA, in psoriasis and provide novel molecular targets for therapeutic purposes.
Keywords: IL-22; keratinocytes; miR-21-3p; miR-21-5p; proliferation; psoriasis.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Wolk K., Haugen H.S., Xu W., Witte E., Waggie K., Anderson M., Vom Baur E., Witte K., Warszawska K., Philipp S., et al. IL-22 and IL-20 are key mediators of the epidermal alterations in psoriasis while IL-17 and IFN-γ are not. J. Mol. Med. 2009;87:523–536. doi: 10.1007/s00109-009-0457-0. - DOI - PubMed
-
- Eyerich S., Eyerich K., Pennino D., Carbone T., Nasorri F., Pallotta S., Cianfarani F., Odorisio T., Traidl-Hoffmann C., Behrendt H., et al. Th22 cells represent a distinct human T cell subset involved in epidermal immunity and remodeling. J. Clin. Investig. 2009;119:3573–3585. doi: 10.1172/JCI40202. - DOI - PMC - PubMed
-
- Wolk K., Witte E., Wallace E., Döcke W.D., Kunz S., Asadullah K., Volk H.D., Sterry W., Sabat R. IL-22 regulates the expression of genes responsible for antimicrobial defense, cellular differentiation, and mobility in keratinocytes: A potential role in psoriasis. Eur. J. Immunol. 2006;36:1309–1323. doi: 10.1002/eji.200535503. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

