Formins in Human Disease
- PMID: 34685534
- PMCID: PMC8533766
- DOI: 10.3390/cells10102554
Formins in Human Disease
Abstract
Almost 25 years have passed since a mutation of a formin gene, DIAPH1, was identified as being responsible for a human inherited disorder: a form of sensorineural hearing loss. Since then, our knowledge of the links between formins and disease has deepened considerably. Mutations of DIAPH1 and six other formin genes (DAAM2, DIAPH2, DIAPH3, FMN2, INF2 and FHOD3) have been identified as the genetic cause of a variety of inherited human disorders, including intellectual disability, renal disease, peripheral neuropathy, thrombocytopenia, primary ovarian insufficiency, hearing loss and cardiomyopathy. In addition, alterations in formin genes have been associated with a variety of pathological conditions, including developmental defects affecting the heart, nervous system and kidney, aging-related diseases, and cancer. This review summarizes the most recent discoveries about the involvement of formin alterations in monogenic disorders and other human pathological conditions, especially cancer, with which they have been associated. In vitro results and experiments in modified animal models are discussed. Finally, we outline the directions for future research in this field.
Keywords: actin; cancer; developmental defects; formins; gene variants; genetic disease.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
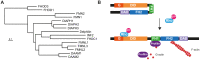



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

