The Intersection of Purine and Mitochondrial Metabolism in Cancer
- PMID: 34685583
- PMCID: PMC8534091
- DOI: 10.3390/cells10102603
The Intersection of Purine and Mitochondrial Metabolism in Cancer
Abstract
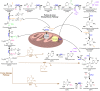
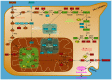
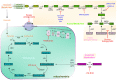
Nucleotides are essential to cell growth and survival, providing cells with building blocks for DNA and RNA, energy carriers, and cofactors. Mitochondria have a critical role in the production of intracellular ATP and participate in the generation of intermediates necessary for biosynthesis of macromolecules such as purines and pyrimidines. In this review, we highlight the role of purine and mitochondrial metabolism in cancer and how their intersection influences cancer progression, especially in ovarian cancer. Additionally, we address the importance of metabolic rewiring in cancer and how the evolving landscape of purine synthesis and mitochondria inhibitors can be potentially exploited for cancer treatment.
Keywords: amino acids; cancers; metabolic reprogramming; mitochondrial metabolism; purines.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Zhao H., Chiaro C.R., Zhang L., Smith P.B., Chan C.Y., Pedley A.M., Pugh R.J., French J.B., Patterson A.D., Benkovic S.J. Quantitative Analysis of Purine Nucleotides Indicates That Purinosomes Increase de Novo Purine Biosynthesis. J. Biol. Chem. 2015;290:6705–6713. doi: 10.1074/jbc.M114.628701. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

