Ventricular Arrhythmias in Ischemic Cardiomyopathy-New Avenues for Mechanism-Guided Treatment
- PMID: 34685609
- PMCID: PMC8534043
- DOI: 10.3390/cells10102629
Ventricular Arrhythmias in Ischemic Cardiomyopathy-New Avenues for Mechanism-Guided Treatment
Abstract
Ischemic heart disease is the most common cause of lethal ventricular arrhythmias and sudden cardiac death (SCD). In patients who are at high risk after myocardial infarction, implantable cardioverter defibrillators are the most effective treatment to reduce incidence of SCD and ablation therapy can be effective for ventricular arrhythmias with identifiable culprit lesions. Yet, these approaches are not always successful and come with a considerable cost, while pharmacological management is often poor and ineffective, and occasionally proarrhythmic. Advances in mechanistic insights of arrhythmias and technological innovation have led to improved interventional approaches that are being evaluated clinically, yet pharmacological advancement has remained behind. We review the mechanistic basis for current management and provide a perspective for gaining new insights that centre on the complex tissue architecture of the arrhythmogenic infarct and border zone with surviving cardiac myocytes as the source of triggers and central players in re-entry circuits. Identification of the arrhythmia critical sites and characterisation of the molecular signature unique to these sites can open avenues for targeted therapy and reduce off-target effects that have hampered systemic pharmacotherapy. Such advances are in line with precision medicine and a patient-tailored therapy.
Keywords: action potential; arrhythmias; calcium; cardiac remodelling; fibrosis; hypertrophy; myocardial infarction.
Conflict of interest statement
The authors report no conflicts of interest.
Figures

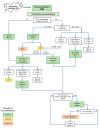



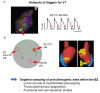
References
-
- Virani S.S., Alonso A., Benjamin E.J., Bittencourt M.S., Callaway C.W., Carson A.P., Chamberlain A.M., Chang A.R., Cheng S., Delling F.N., et al. Heart Disease and Stroke Statistics—2020 Update: A Report from the American Heart Association. Circulation. 2020;141:e139–e596. doi: 10.1161/CIR.0000000000000757. - DOI - PubMed
-
- Berg D.D., Wiviott S.D., Braunwald E., Guo J., Im K., Kashani A., Gibson C.M., Cannon C.P., Morrow D.A., Bhatt D.L., et al. Modes and timing of death in 66 252 patients with non-ST-segment elevation acute coronary syndromes enrolled in 14 TIMI trials. Eur. Heart J. 2018;39:3810–3820. doi: 10.1093/eurheartj/ehy556. - DOI - PMC - PubMed
-
- Solomon S.D., Zelenkofske S., Mcmurray J., Finn P.V., Velazquez E., Ertl G., Harsanyi A., Rouleau J.L., Maggioni A.P., Kober L., et al. Sudden Death in Patients with Myocardial Infarction and Left Ventricular Dysfunction, Heart Failure, or Both. N. Engl. J. Med. 2005;352:2581–2588. doi: 10.1056/NEJMoa043938. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

