Transcriptomic Biomarkers for Tuberculosis: Validation of NPC2 as a Single mRNA Biomarker to Diagnose TB, Predict Disease Progression, and Monitor Treatment Response
- PMID: 34685683
- PMCID: PMC8534371
- DOI: 10.3390/cells10102704
Transcriptomic Biomarkers for Tuberculosis: Validation of NPC2 as a Single mRNA Biomarker to Diagnose TB, Predict Disease Progression, and Monitor Treatment Response
Abstract
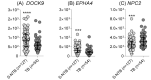
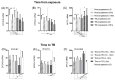
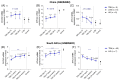
External validation in different cohorts is a key step in the translational development of new biomarkers. We previously described three host mRNA whose expression in peripheral blood is significantly higher (NPC2) or lower (DOCK9 and EPHA4) in individuals with TB compared to latent TB infection (LTBI) and controls. We have now conducted an independent validation of these genes by re-analyzing publicly available transcriptomic datasets from Brazil, China, Haiti, India, South Africa, and the United Kingdom. Comparisons between TB and control/LTBI showed significant differential expression of all three genes (NPC2high p < 0.01, DOCK9low p < 0.01, and EPHA4low p < 0.05). NPC2high had the highest mean area under the ROC curve (AUROC) for the differentiation of TB vs. controls (0.95) and LTBI (0.94). In addition, NPC2 accurately distinguished TB from the clinically similar conditions pneumonia (AUROC, 0.88), non-active sarcoidosis (0.87), and lung cancer (0.86), but not from active sarcoidosis (0.66). Interestingly, individuals progressing from LTBI to TB showed a constant increase in NPC2 expression with time when compared to non-progressors (p < 0.05), with a significant change closer to manifestation of active disease (≤3 months, p = 0.003). Moreover, NPC2 expression normalized with completion of anti-TB treatment. Taken together, these results validate NPC2 mRNA as a diagnostic host biomarker for active TB independent of host genetic background. Moreover, they reveal its potential to predict progression from latent to active infection and to indicate a response to anti-TB treatment.
Keywords: Mycobacterium tuberculosis; NPC2; Niemann–Pick disease type C2; RNA; biomarkers; diagnosis; mRNA; transcription; treatment; tuberculosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- WHO . Global Tuberculosis Report. WHO; Geneva, Switzerland: 2020.
-
- Gocmen O., Babayigit C., Ozer B., Inandi T., Ozer C., Duran N. Performance of QuantiFERON-TB Gold In-Tube test and Tuberculin Skin Test for diagnosis of latent tuberculosis infection in BCG vaccinated health care workers. Med. Sci. Monit. 2014;20:521–529. doi: 10.12659/MSM.889943. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

