Redox Control of the Dormant Cancer Cell Life Cycle
- PMID: 34685686
- PMCID: PMC8535080
- DOI: 10.3390/cells10102707
Redox Control of the Dormant Cancer Cell Life Cycle
Abstract
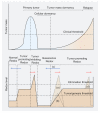
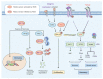
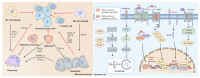
Following efficient tumor therapy, some cancer cells may survive through a dormancy process, contributing to tumor recurrence and worse outcomes. Dormancy is considered a process where most cancer cells in a tumor cell population are quiescent with no, or only slow, proliferation. Recent advances indicate that redox mechanisms control the dormant cancer cell life cycle, including dormancy entrance, long-term dormancy, and metastatic relapse. This regulatory network is orchestrated mainly through redox modification on key regulators or global change of reactive oxygen species (ROS) levels in dormant cancer cells. Encouragingly, several strategies targeting redox signaling, including sleeping, awaking, or killing dormant cancer cells are currently under early clinical evaluation. However, the molecular mechanisms underlying redox control of the dormant cancer cell cycle are poorly understood and need further exploration. In this review, we discuss the underlying molecular basis of redox signaling in the cell life cycle of dormant cancer and the potential redox-based targeting strategies for eliminating dormant cancer cells.
Keywords: ROS; cancer dormancy; cancer therapy; redox signaling.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Tian H., Zhang J., Zhang H., Jiang Y., Song A., Luan Y. Low side-effect and heat-shock protein-inhibited chemo-phototherapy nanoplatform via co-assembling strategy of biotin-tailored IR780 and quercetin. Chem. Eng. J. 2020;382:123043. doi: 10.1016/j.cej.2019.123043. - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

